The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule
Maryam Oroujeni1, Tianqi Xu1, Katherine Gagnon2,3, Sara S. Rinne3, Jan Weis4, Javad Garousi1, Ken G. Andersson5, John Löfblom5, Anna Orlova3,6, Vladimir Tolmachev1,6
Myocardial perfusion recovery induced by an a-calcitonin gene-related peptide analogue
Simon Bentsen, MD1, Anette Sams, PhD2, Philip Hasbak, MD, DMSc1, Lars Edvinsson, MD, PhD, DMSc2, Andreas Kjaer, MD, PhD, DMSc1, Rasmus S. Ripa, DMSc1
1Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark
2Department of Clinical Experimental Research, Glostrup Research Institute, Glostrup University Hospital, Glostrup, Denmark
https://doi.org/10.1007/s12350-021-02678-8
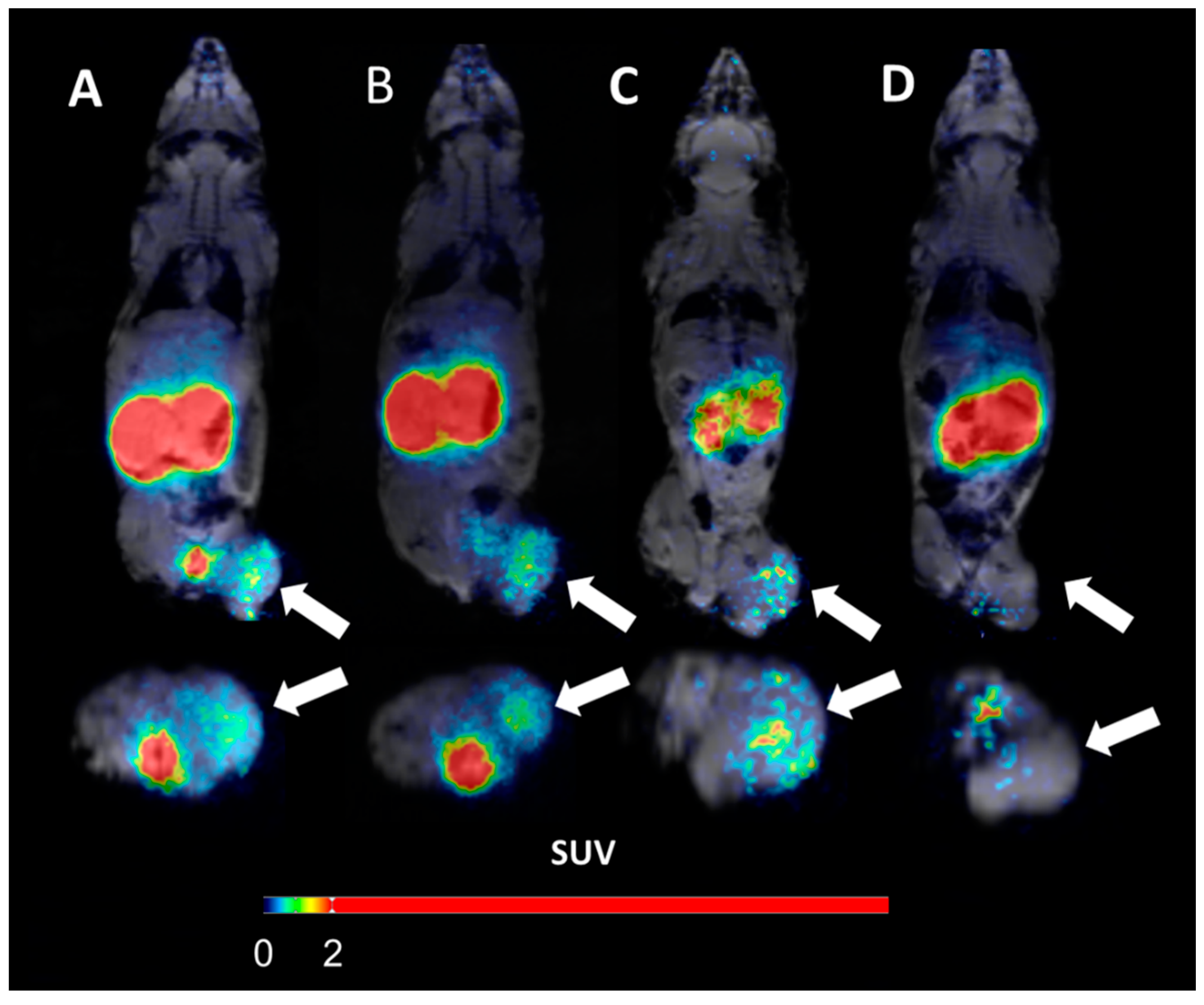
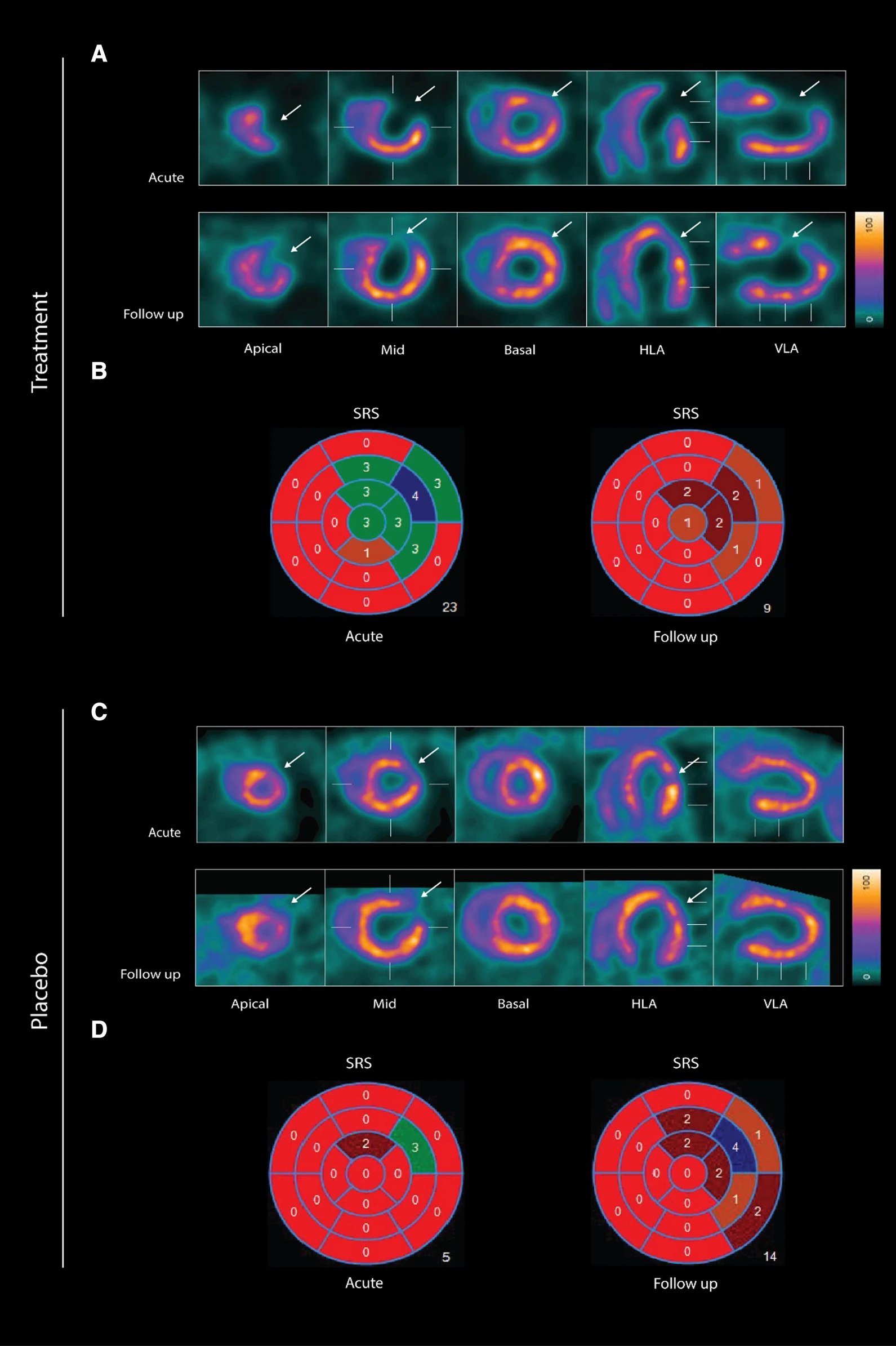
wo representative examples of rats with chronic LAD occlusion from the SPECT/CT scans. 4DM software was used to analyze [99mTc]Tc-sestamibi SPECT/CT. Top panels (A and B) show a SAX-treated rat. Bottom panels (C and D) show a placebo-treated rat.(A) Large perfusion defect in the anterior and apical wall in the acute scan, with a smaller perfusion defect at follow-up scan after SAX treatment (white arrow).(B) The perfusion defects from panel A in a 17-segment polar map.(C) Medium perfusion defect in the anterior wall at the acute scan, and a more severe perfusion defect at follow-up after placebo treatment (red arrow).(D) The perfusion defects from panel C in a 17-segment polar map. HLA horizontal long axis, VLA vertical long axis, SRS summed rest score.
- The results show that an analogue of CGRP that induces both coronary and peripheral vasodilation significantly improves myocardial perfusion recovery after experimental myocardial infarction in rats. There was no significant difference in overall survival between the two groups suggesting that SAX does not have a damaging effect on the animals or the myocardium.
- In conclusion, the CGRP analogue, SAX, seems to have a cardioprotective effect on a rat model of myocardial infarction, by improving the perfusion recovery after a chronic occlusion of the coronary artery.
Bispecific GRPR-Antagonistic Anti-PSMA/GRPR Heterodimer for PET and SPECT Diagnostic Imaging of Prostate Cancer
Bogdan Mitran1, Zohreh Varasteh1,2, Ayman Abouzayed1, Sara S. Rinne1, Emmi Puuvuori1, Maria De Rosa1,3, Mats Larhed1,4, Vladimir Tolmachev5, Anna Orlova1,4, Ulrika Rosenström1
1Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
2Department of Nuclear Medicine, Klinikum rechts der Isar der TUM, 81675 Munich, German
3Drug Discovery Unit, Ri.MED Foundation, 90133 Palermo, Italy
4Science for Life Laboratory, Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
5Department of Immunology, Genetics and Pathology, Uppsala University, 751 23 Uppsala, Sweden
https://doi.org/10.3390/cancers11091371
Whole body SPECT/CT scans of the mice bearing PC3-PIP xenografts injected with 111In-6 (400 kBq, 100 pmol/mouse) were performed using nanoScan SPECT/CT at 1, 3, and 24 h pi. Additionally, three mice were co-injected with either non-labeled PSMA-617 (1.5 nmol), non-labeled NOTA-PEG6-RM26 (1.5 nmol), or a combination of both, and imaged 1 h pi. CT scans were acquired at the following parameters: 50 keV, 670 μA, 480 projections, and 5 min acquisition time. SPECT scans were carried out using 111In energy windows (154 keV–188 keV; 221 keV–270 keV), a 256 × 256 matrix, and a 30 min acquisition time. The CT raw data were reconstructed using Nucline 2.03 Software. SPECT raw data were reconstructed using Tera-Tomo™ 3D SPECT.
PET/CT scans of the mice injected with 68Ga-6 (1.8 MBq, 100 pmol/mouse) were performed using nanoScan PET/MRI 3T at 1 h pi. To evaluate the in vivo specificity, one mouse was co-injected with a combination of non-labeled PSMA-617 (1.5 nmol) and non-labeled NOTA-PEG6-RM26 (1.5 nmol). CT acquisitions were performed as previously described using nanoScan SPECT/CT immediately after PET acquisition employing the same bed position. PET scans were performed for 30 min. PET data were reconstructed into a static image using the Tera-Tomo™ 3D reconstruction engine. CT data were reconstructed using Filter Back Projection. PET and CT files were fused and analyzed using Nucline 2.03 Software.
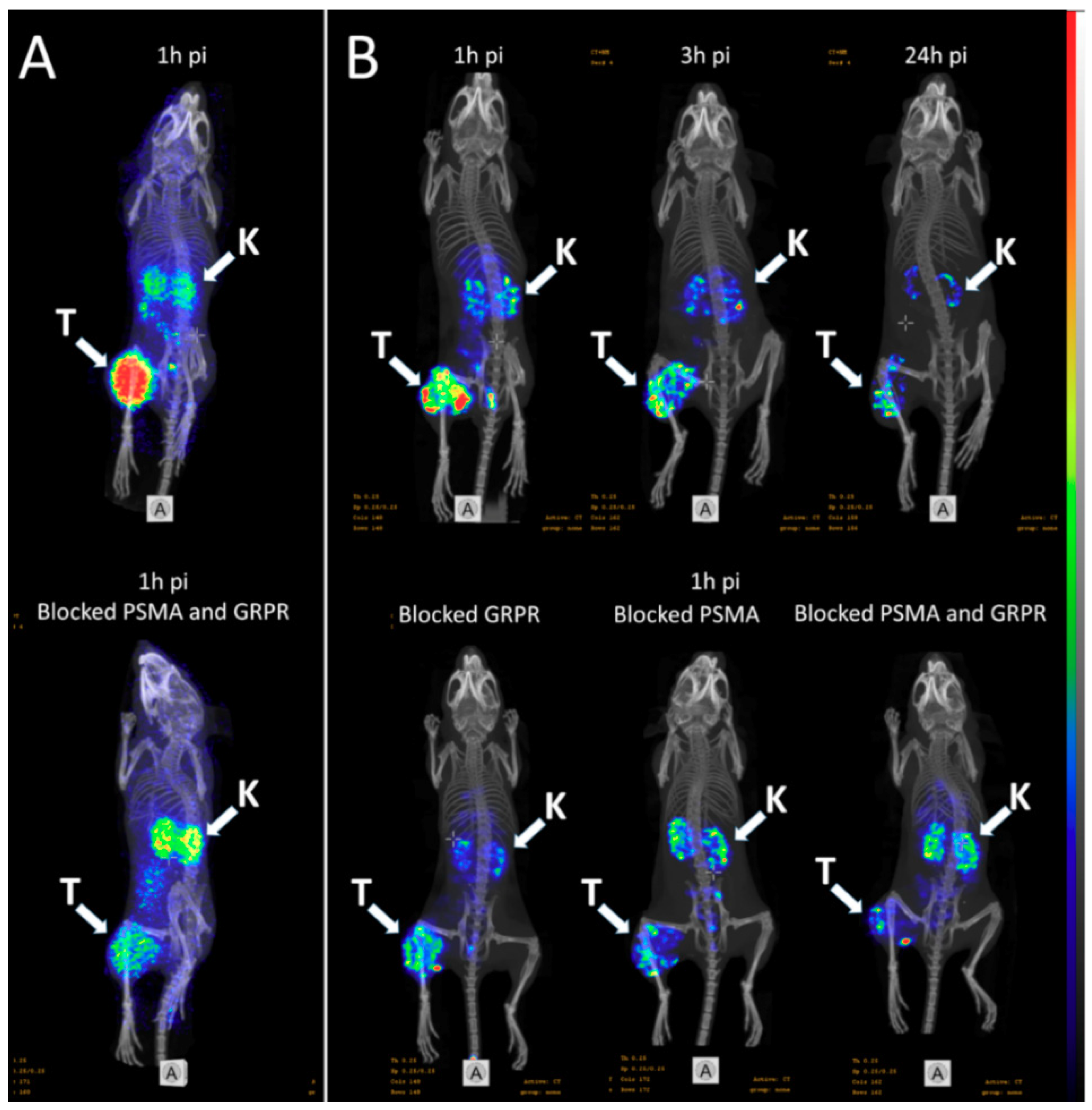
(A) micro positron emission tomography (microPET)/CT and (B) microSPECT/CT MIP images of PC3-PIP-xenografted mice (PSMA positive/GRPR positive) after an iv injection of 68Ga-6 and 111In-6. Blocked animals were co-injected with PSMA-617 for the blocking of PSMA and RM26 for the blocking of GRPR, or both.
- The imaging of PC3-PIP tumors using microPET/CT for 68Ga-6 (1 h pi) and microSPECT/CT for 111In-6 (1, 3, and 24 h pi) confirmed the ex vivo biodistribution data. Tumors were clearly visualized at all time-points, while weak activity accumulation was only observed in the kidneys among healthy organs. In agreement with ex vivo measurements, imaging contrast improved with time up to 3 h pi, despite the rapid washout of activity from tumors. The superior imaging contrast obtained for 111In-6 compared to 68Ga-6 was also in agreement with the biodistribution data. The imaging of mice after the co-injection of excess PSMA- and GRPR-targeting monomers corroborated with the ex vivo biodistribution pattern: partially decreased activity uptake in tumors, but increased activity uptake in kidneys, were exhibited in the case of PSMA blocking.
- MicroPET/CT and microSPECT/CT scans confirmed biodistribution data, suggesting that 68Ga-NOTA-DUPA-RM26 and 111In-NOTA-DUPA-RM26 are suitable candidates for the imaging of GRPR and PSMA expression in PCa shortly after administration.
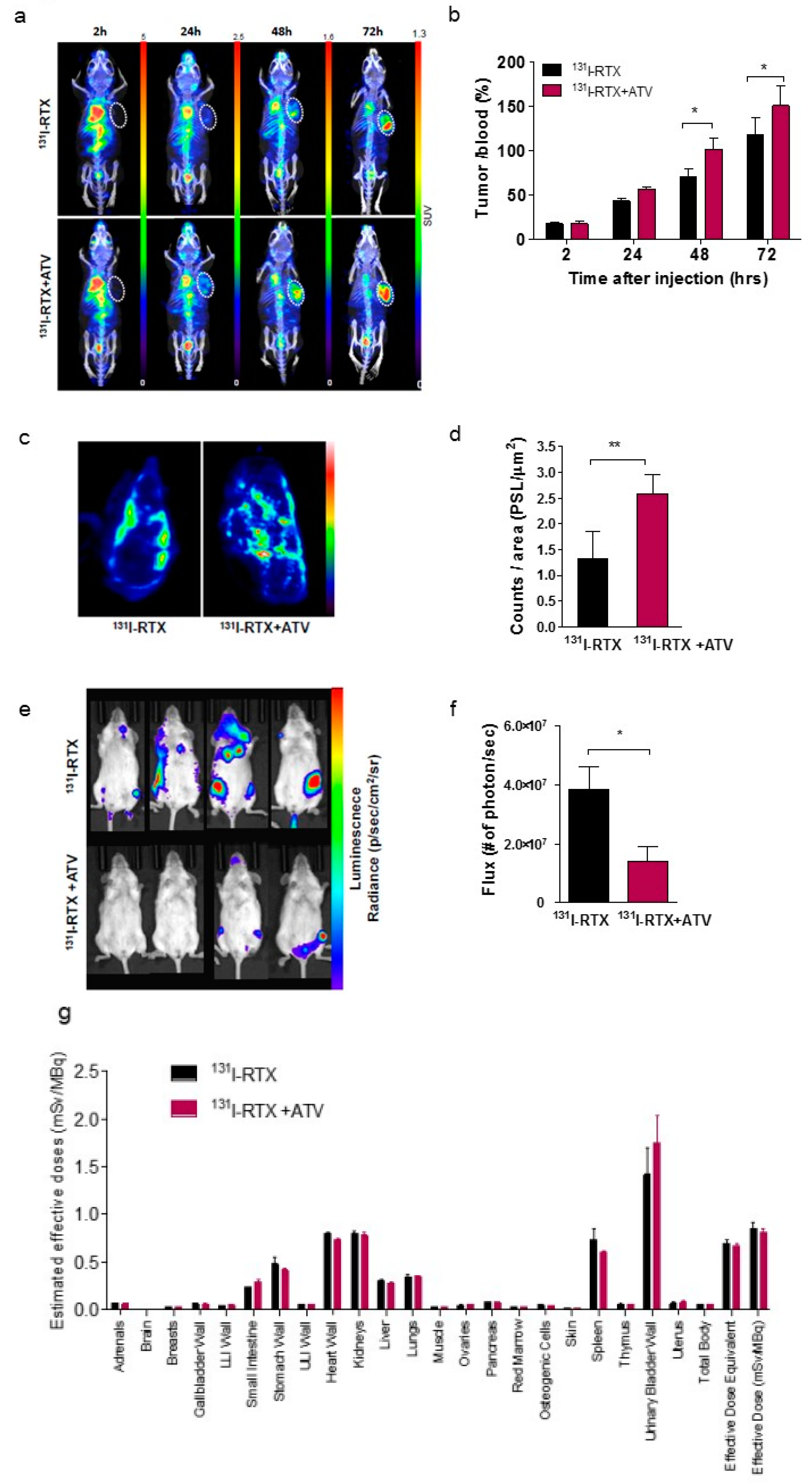
Inhibition of HIF-1α by Atorvastatin During 131I-RTX Therapy in Burkitt’s Lymphoma Model
Eun-Ho Kim1,2, Hae Young Ko3,4, A Ram Yu5, Hyeongi Kim3, Javeria Zaheer3,6, Hyun Ji Kang3,6, Young-Cheol Lim3, Kyung Deuk Cho3, Hyun-Yoo Joo1, Min Kyoung Kang5, Jae Jun Lee5, Seung-Sook Lee7, Hye Jin Kang8, Sang Moo Lim3,9, Jin Su Kim3,6
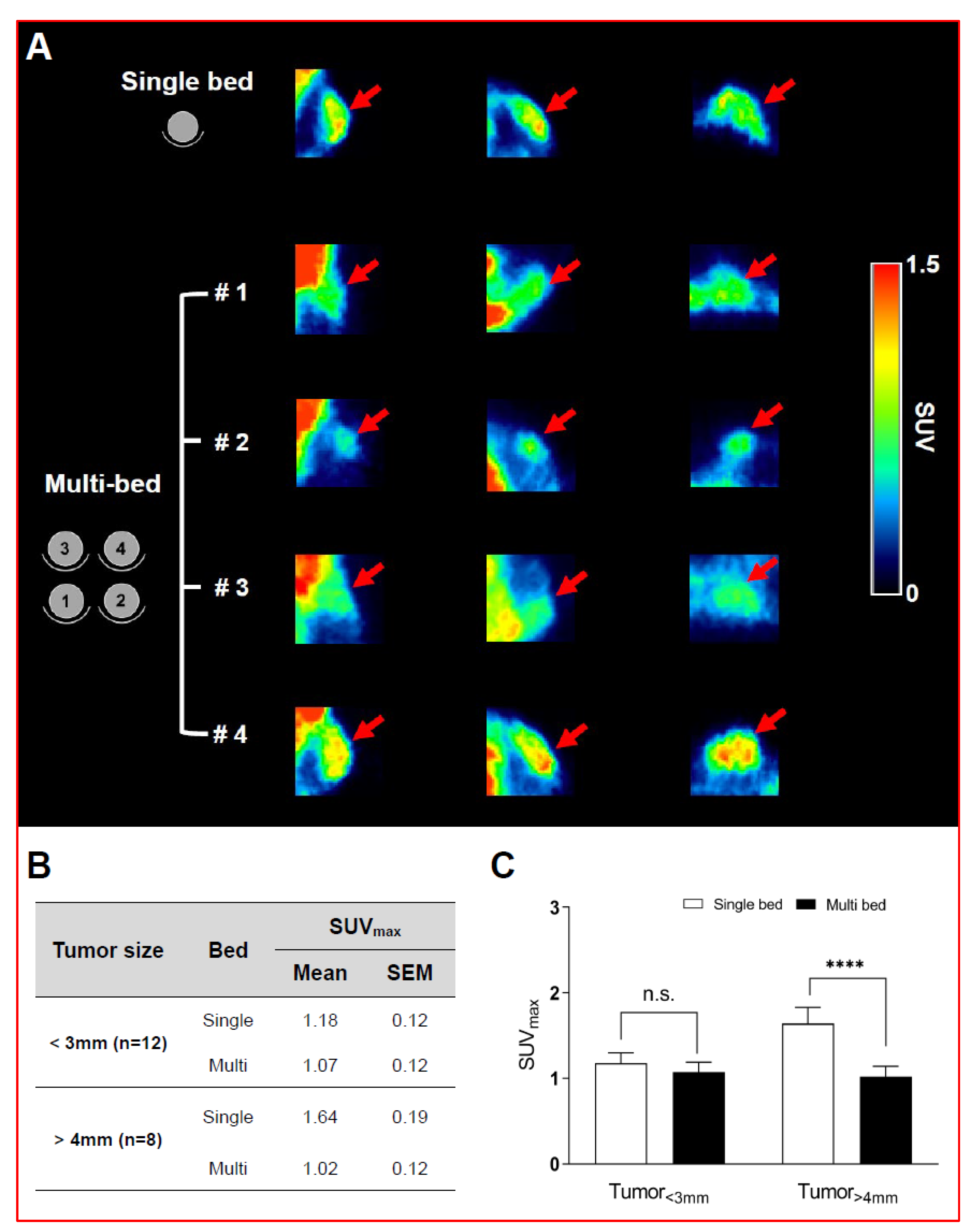
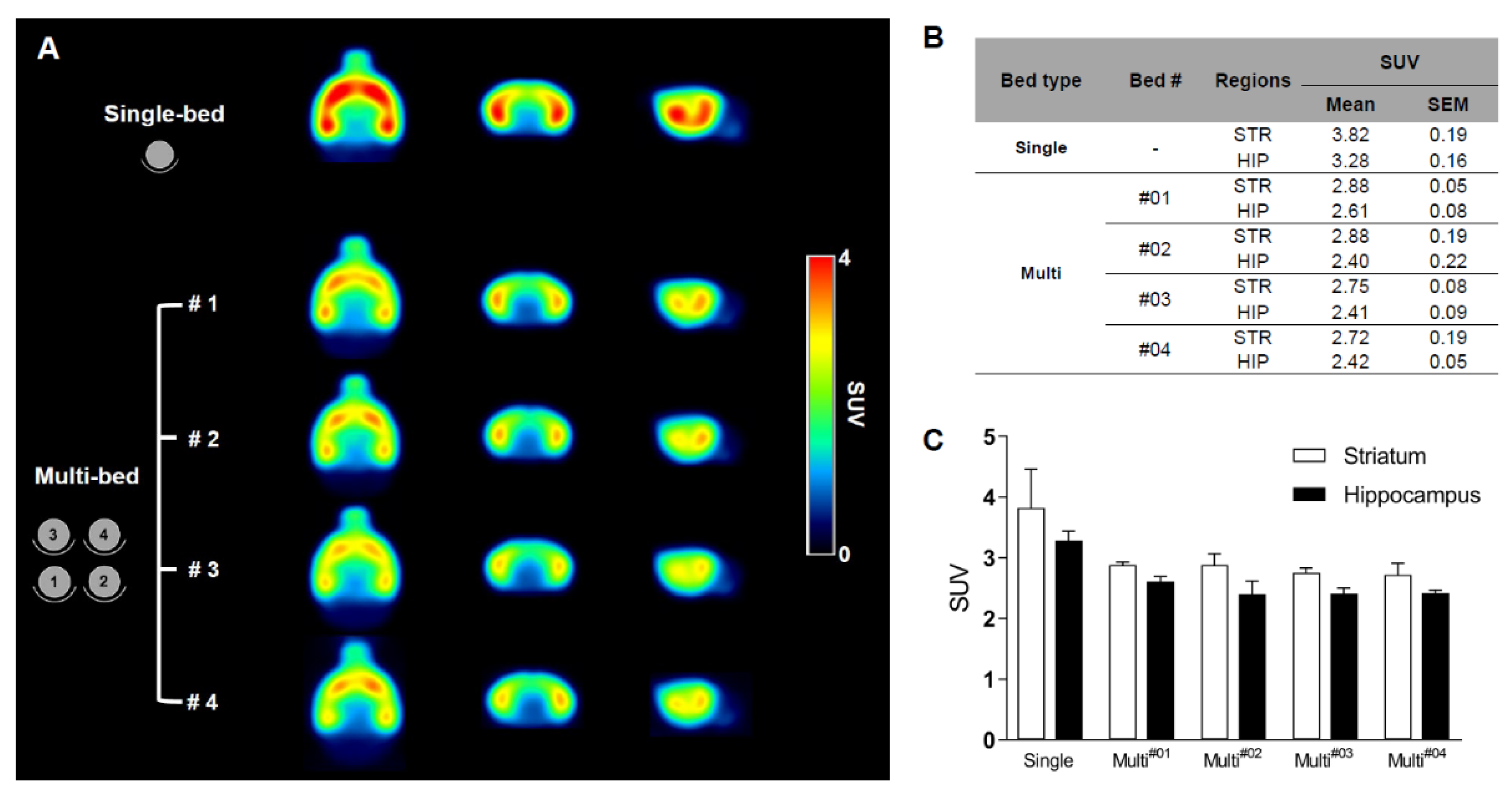
Validation of Image Qualities of a Novel Four-Mice Bed PET System as an Oncological and Neurological Analysis Tool
Kyung Jun Kang1, Se Jong Oh1, Kyung Rok Nam1, Heesu Ahn1,2, Ji-Ae Park1,2, Kyo Chul Lee1, Jae Yong Choi1,2
1Division of Applied RI, Korea Institute of Radiological and Medical Sciences, Seoul 01812, Korea
2Radiological and Medico-Oncological Sciences, University of science and technology (UST), Seoul 01812, Korea
https://doi.org/10.3390/jimaging7030043
For this purpose, researchers use micro-PET (µPET), which is a small-animal dedicated system. Most µPET vendors provide a single bed, thereby allowing imaging of only a single animal at a time. Large-scale research involving many objects thus requires tremendous time and use of radioactivity.
Recently, Mediso developed a multi-bed system dedicated to the nanoScan scanners with the contribution of Tim Witney and his team at UCL and KCL, where the initial validity for research has been investigated. Greenwood et al. (https://doi.org/10.2967/jnumed.119.228692) tested the four-bed mouse system using 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) in phantom and normal mice and reported a quantitative accuracy similar to that of a single-bed. To date, however, few studies have focused on the validity of oncological and neurological PET imaging of the four-mice bed system.
This study aimed to evaluate the image qualities of oncological and neurological PET imaging using a novel four-mice bed system.
In vivo imaging with 18F-FDG- and 18F-Florbetaben-PET/MRI detects pathological changes in the brain of the commonly used 5XFAD mouse model of Alzheimer's Disease
Iodine-124 PET quantification of organ-specific delivery and expression of NIS-encoding RNA
Matthias Miederer1, Stefanie Pektor1, Isabelle Miederer1, Nicole Bausbacher1, Isabell Sofa Keil2, Hossam Hefesha3, Heinrich Haas3, Ugur Sahin2,3, Mustafa Diken2,3
1Department of Nuclear Medicine, University Medical Center of Johannes Gutenberg University, Mainz, Germany
2TRON - Translational Oncology at the University Medical Center, Johannes Gutenberg University Mainz gGmbH, Mainz, Germany
3Biopharmaceutical New Technologies (BioNTech) SE, Mainz, Germany
https://doi.org/10.1186/s13550-021-00753-2
Summary
RNA-based vaccination strategies tailoring immune response to specific reactions have become an important pillar for a broad range of applications. Recently, the use of lipid-based nanoparticles opened the possibility to deliver RNA to specific sites within the body, overcoming the limitation of rapid degradation in the bloodstream.
Nanoparticles show promising potency as delivery vehicles for a variety of molecules, leading to fundamentally new applications and therapeutic strategies. Due to their complex chemical composition and relevant interaction with plasma proteins, the pharmacokinetic properties and delivery properties of nanoparticles are variable and remain challenging to adapt for optimal conditions. Accomplishing precise RNA delivery to target tissue using nanoparticles would serve as a versatile platform that enables easy and transient expression of any protein in principal. RNA is currently already in use to selectively activate the immune system against specific target proteins for cancer therapy.
In the article, the authors have investigated whether small animal PET/MRI can be employed to image the biodistribution of RNA-encoded protein. For this purpose, a reporter RNA coding for the sodium-iodide-symporter (NIS) was assembled with liposomes at different charge ratios, and functional NIS protein translation was imaged and quantified in vivo and ex vivo by Iodine-124 PET upon intravenous administration in mice.
Results from the nanoScan PET/MRI
For the small animal imaging, the authors have used a nanoScan PET/MRI 1T, which provided a perfect option to follow the uptake of Iodine-124 not just in the thyroids, but also in the NIS-expressing tissues. Moreover, it could be accompanied by the help of MRI, to identify internal organs, like spleen.
Groups of n = 3 animals were intravenously injected with RNA-lipoplexes containing 20 µg NIS RNA. Six hours later 6.64 ± 0.66 MBq Iodine-124 was injected intravenously. Three hours after Iodine-124 injection, mice were anesthetized with 2% isoflurane and static imaging was performed over 20 min. For anatomic imaging MRI measurements (Material Map for coregistration of the PET scan; 3D Gradient Echo External Averaging (GRE-EXT), Multi Field of View (FOV); slice thickness: 0.6 mm; TE: 2 ms; TR: 15 ms; flip angle: 25 deg) were performed afterward. Additionally, one animal per group was imaged dynamically for one hour. PET data were reconstructed with Teratomo 3D (4 iterations, 6 subsets, voxel size 0.4 mm), co-registered to the MR and corrected for decay.
Figure 2. shows the PET/MRI of Iodine-124 distribution in vivo. (A) Coronal slices of PET/MRI fusion and volumes of interests (red) for spleen and lung are shown in representative animals. From left to right: targeting of spleen with non-coding RNA, targeting of spleen with NIS RNA, targeting of lung with non-coding RNA, targeting of lung with NIS-RNA. (B) Calculated organ uptake from the volumes of interests. Data are shown as mean + SD of n = 3 mice. (C) Representative in vivo bioluminescence images of Luc-RNA lipoplexes after targeting the spleen and lung. (D) Maximum intensity projections of PET images after application of lung-targeting NIS-RNA lipoplexes (right) in comparison with non-coding control (left). (E) Time activity curve of Iodine-124 uptake in the lung over 60 min immediately after Iodine-124 injection (6 h after administration of NIS-RNA lipoplexes targeting the lung).
- In this study, two RNA-lipoplex systems for systemic NIS-RNA delivery were compared by small animal PET/MRI of Iodine-124 uptake. One system with net anionic charge is known to mediate translation primarily within the spleen, and the other with net positive charge is known to yield translation primarily within the lungs.
- Tha authors have shown highly specific targeting, delivery and expression of RNA to spleen and lung by anionic and cationic RNA-lipoplex nanoparticles, respectively, through the use of the NIS reporter gene system and Iodine-124 uptake as imaged by PET/MRI. Combining NIS reporter gene imaging with in vivo small animal PET/MRI thus represents a powerful tool to monitor the distribution and extent of expression of RNA targeted specifically to any tissue over time.

Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging
Falco Reissig1, David Bauer1,2, Martin Ullrich1, Martin Kreller1, Jens Pietzsch1,2, Constantin Mamat1,2, Klaus Kopka1,2, Hans-Jürgen Pietzsch1, Martin Walther1
1Helmholtz-Zentrum Dresden-Rossendorf, Institut für Radiopharmazeutische Krebsforschung, Bautzner Landstraße 400, D-01328 Dresden, Germany
2Fakultät Chemie und Lebensmittelchemie, Technische Universität Dresden, D-01062 Dresden, Germany
https://doi.org/10.3390/ph13100272
Summary
Barium-131 is a single photon emission computed tomography (SPECT)-compatible radionuclide for nuclear medicine and a promising diagnostic match for Radium-223/-224. In the early 1970s, Barium-131 has been thoroughly investigated as a potential bone targeting radiotracer, but no substantial benefits have been mentioned, comparing it to other already applicable radiotracers like [18F]F− (t½ = 110 min) and 99mTc-labeled (t½ = 6.0 h) bisphosphonates. However, as part of current approaches to the therapy of bone cancer and bone metastases, this radionuclide has its significance in modern times. Barium-131 possesses the suitable half-life of 11.5 d, thereby making it highly beneficial for potential diagnostic use in nuclear medicine. Due to the similar chemistry and pharmacological properties of the elements Barium and Radium, Barium-131 is particularly feasible as a diagnostic match to the therapeutic α-emitters Radium-223 and Radium-224. In the work presented here, the authors aimed to establish a simple but sufficient procedure for the production and purification of n.c.a. Barium-131 using the TR-FLEX cyclotron (ACSI), starting from a cheap Cesium Chloride target with natural monoisotopically occurring Cesium followed by 27.5 MeV proton bombardment. Moreover, the in-house produced Barium-131 was used for first labeling studies with the chelator macropa, for initial in vivo-related phantom studies and, last but not least, small animal imaging trials with [131Ba]Ba(NO3)2 and 131Ba-labeled macropa in healthy mice.
Results from the nanoScan SPECT/CT
For the small animal imaging, the authors have used a nanoScan SPECT/CT, to follow the biodistribution with the different Barium-131 tracers.
SPECT/CT imaging in mice was performed at 1 h and 24 h after i.v. injection of [131Ba]Ba(NO3)2 (6.2 MBq in 0.2 mL of 0.01 M HNO3, pH 6, Am = 420 GBq/µmol, n.c.a.), or 131Ba-labeled macropa (6.7 MBq in 0.2 mL of 0.1 M ammonium acetate, pH 6, Am = 83 MBq/µmol) with a frame time of 60 s (total scan time: 1.5 h), respectively. The acquisition was performed using a standard aperture for mouse imaging (APT62) consisting of four M3 multi-pinhole collimators providing a 30 × 30 mm transaxial field of view (FOV). Projection data were reconstructed using the Tera-Tomo™ 3D high dynamic range algorithm (resolution: 128; iterations: 48; subset size: 4), applying corrections for decay, scatter, and attenuation.
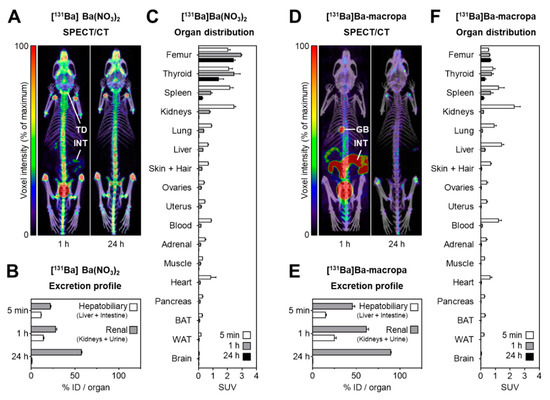
Figure 8. shows the distribution of [131Ba]Ba(NO3)2 and [131Ba]Ba-macropa in mice. (A) SPECT/CT fusion images of [131Ba]Ba(NO3)2 in a mouse 1 h and 24 h after injection; (B,C) excretion profile and organ distribution of [131Ba]Ba(NO3)2 in mice 5 min, 1 h, and 24 h after injection (n = 4); (D) SPECT/CT fusion images of [131Ba]Ba-macropa in a mouse 1 h and 24 h after injection; (E,F) excretion profile and organ distribution of [131Ba]Ba-macropa in mice; 5 min, 1 h, and 24 h after injection (n = 4); (BAT) brown adipose tissue; (GB) gall bladder *; (ID) initial dose; (INT) intestine; (TD) thyroid/parathyroid *; (WAT) white adipose tissue (* activity in these organs was not measured separately).
- The author have shown for the first time the in vivo biodistribution behavior of 131Ba-labeled macropa in comparison with free [131Ba]Ba2+ by means of small animal SPECT/CT.
- Biodistribution studies revealed the expected rapid bone uptake of [131Ba]Ba2+, whereas 131Ba-labeled macropa showed a fast clearance from the blood, thereby showing a significantly (p < 0.001) lower accumulation in the bone. The authors have concluded that barium-131 is a promising SPECT radionuclide and delivers appropriate imaging qualities in small animals. Furthermore, the relative stability of the 131Ba-labeled macropa complex in vivo forms the basis for the development of sufficient new chelators, especially for radium isotopes. Thereby, barium-131 will attain its goal as a diagnostic match to the alpha emitters radium-223 and radium-224.
Indium-111-labeled CD166-targeted peptide as a potential nuclear imaging agent for detecting colorectal cancer stemlike cells in a xenograft mouse model
Siao-Syun Guan1, Cheng-Tien Wu2,3, Tse-Zung Liao1, Tsai-Yueh Luo1, Kun-Liang Lin1, Shing-Hwa Liu4,5,6
1Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan
2Department of Nutrition, China Medical University, Taichung, 40402 Taiwan
3Master Program of Food and Drug Safety, China Medical University, Taichung, 40402 Taiwan
4Institute of Toxicology, College of Medicine, National Taiwan University, No. 1, Jen-Ai Road, Section 1, Taipei, 10051 Taiwan
5Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan
6Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan
https://dx.doi.org/10.1186%2Fs13550-020-0597-3
Summary
Colorectal cancer (CRC) is the third most frequent occurring cancer in men and the second most frequent occurring cancer in women, with nearly 1.65 million new diagnosed cases and about 832,000 deaths in 2015. One possible cause of treatment failure is that the tumor site contains a small population of tumor-initiating cells termed cancer stem cells (CSCs). CSCs are involved in drug resistance, metastasis, and relapse of cancers, which can significantly affect tumor therapy. Hence, to develop specifically therapeutic target probe at CSCs for improvement of survival and quality of life of cancer patients is urgently needed. The CD166 protein has been suggested to be involved in CRC tumorigenesis and to be considered a marker for colorectal CSCs (CRCSCs) detection. In this study, therefore, the authors attend to apply a nuclear imaging agent probe, Glycine18-Cystine-linked CD166-targeted peptides (CD166tp-G18C), to detect the changes of CD166 level in a CRC xenograft mouse model.
Results from the nanoSPECT/CT
For the animal experiment, the authors have used a nanoSPECT/CT, which provided a good enough sensitivity and resolution to make the tumor uptake visible after 2 hours p.i., and see significant differences in the uptake of the applied tracers.
To create a xenograft tumor model, male BALB/c nude mice were subcutaneously inoculating CD166+HCT15 cells (1 × 106 cells) for 2 weeks, and then the 111In-DTPA, 111In-DTPA-G18C, 111In-DTPA-CD166tp-C, and 111In-DTPA-CD166tp-G18C (740 MBq/kg/mouse) were intravenously injected into mice. The imaging of CD166 in mice at 2, 4, 24, and 48 h were detected by the nanoSPECT/CT. For competitive study, the CD166+HCT15-derived xenograft mice were pre-treated with CD166tp-G18C (0, 10, and 50 mg/kg) for 6 h. Every mouse then received 740 MBq/kg 111In-DTPA-CD166tp-G18C via intravenous injection for 24 and 48 h. The competitive CD166 images were observed with the same procedure.
Figure 8. shows the main results from the SPECT/CT acquisitions: The nuclear imaging tracer of 111In-DTPA-CD166tp-G18C for detection of CD166-positive colorectal tumor in vivo. a) The colorectal tumor nuclear imaging analysis in CD166+HCT15 xenograft mice. The 111In-DTPA-CD166tp-G18C and control groups (740 MBq/kg/per mouse) were intravenously injected into mice for 2, 4, 24, and 48 h and detected by a nanoSPECT/CT. Group I, 111In-DTPA; Group II, 111In-DTPA-G18C; Group III, 111In-DTPA-CD166tp-C; Group IV, 111In-DTPA-CD166tp-G18C. b) Quantification of nuclear images in tumor areas of colorectal tumor xenograft mice. The circled positions in images were quantified by a 3D analysis software. Data are presented as mean ± SD (n ≥ 3). *P < 0.05, versus control group. c) The competitive study of 111In-DTPA-CD166tp-G18C in CD166+HCT15 xenograft mice. After tumor xenograft mice were intravenously injected with CD166tp-G18C (0, 10, and 50 mg/kg) for 6 h, 111In-DTPA-CD166tp-G18C (740 MBq/kg/mouse) was intravenously injected into mice for 24 and 48 h and detected by a nanoSPECT/CT. d) Quantification of nuclear images in tumor areas of colorectal tumor xenograft mice. Data are presented as mean ± SD (n ≥ 3). *P < 0.05, versus 0 mg/kg CD166tp-G18C group, #P < 0.05, versus 0 mg/kg CD166tp-G18C group.
- The authors have developed a nuclear imaging agent (111In-DTPA-CD166tp-G18C) using CD166tp-G18C as a probe for CD166-positive CRCs detection in a xenograft mouse model. In this xenograft model, when the tumor size achieved about 150 mm3 which possessed about 1 × 107 CD166-postive cells (cancer cell average diameter: 15 μm), the nanoSPECT/CT detection started to perform.
- These results suggest that CD166-positive CRC exhibited characteristics of CSCs, so it may be a useful drug screening tool for CRC diagnosis. The authors synthesized DTPA-CD166tp-G18C and radiolabeled with Indium-111 for detecting CD166 imaging by using nanoSPECT/CT in CD166-positive CRC xenograft mice. The bio-distribution of 111In-DTPA-CD166tp-G18C confirmed the accumulation of CD166-positive cells in tumors. Therefore, 111In-DTPA-CD166tp-G18C may be a potential nuclear imaging agent for diagnosis of CRCSCs. The CD166 bound peptide-based nuclear imaging may provide physicians to classify cancer cells before treatment and monitor patients with a history of CRC after surgery or drug treatment.

Opioid–galanin receptor heteromers mediate the dopaminergic effects of opioids
Ning-Sheng Cai1, César Quiroz1, Jordi Bonaventura2, Alessandro Bonifazi3, Thomas O. Cole4, Julia Purks5, Amy S. Billing6, Ebonie Massey6, Michael Wagner6, Eric D. Wish6, Xavier Guitart1, William Rea1, Sherry Lam2, Estefanía Moreno7, Verònica Casadó-Anguera7, Aaron D. Greenblatt4, Arthur E. Jacobson8, Kenner C. Rice8, Vicent Casadó7, Amy H. Newman3, John W. Winkelman5, Michael Michaelides2, Eric Weintraub4, Nora D. Volkow9, Annabelle M. Belcher4, Sergi Ferré1
1Integrative Neurobiology Section.
2Biobehavioral Imaging and Molecular Neuropsychopharmacology Unit and.
3Medicinal Chemistry Section, National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP), NIH, Baltimore, Maryland, USA.
4Division of Alcohol and Drug Abuse, Department of Psychiatry, School of Medicine, University of Maryland, Baltimore, Maryland, USA.
5Massachusetts General Hospital, Departments of Psychiatry and Neurology, Harvard Medical School, Boston, Massachusetts, USA.
6Center for Substance Abuse Research, University of Maryland, College Park, Maryland, USA.
7Department of Biochemistry and Molecular Biomedicine, University of Barcelona, Barcelona, Spain.
8Drug Design and Synthesis Section, NIDA, IRP, and.
9NIDA, NIH, Baltimore, Maryland, USA.
https://doi.org/10.1172/jci126912
Summary
Identifying nonaddictive opioid medications is a high priority in medical science, but μ-opioid receptors (MORs) mediate both the analgesic and addictive effects of opioids. And as possibly everyone knows, the opioid epidemic shows a severe public health crisis worldwide.
Maintenance treatment with the (MOR) agonist methadone is the most highly researched and evidence-based treatment for opioid use disorder. Yet public perception concerning the substitution of illicit drugs (such as heroin) with medication (such as methadone) has led to stigmatized views of maintenance treatment, stalling the advancement of addiction treatment policy and access to medication-based treatments. MOR agonism also offers the most effective treatment for severe pain, making the search for a nonaddictive opioid drug the holy grail of pain research.
The neuropeptide galanin acts as a modulator of neurotransmission in the CNS and the PNS, and It is coexpressed with different neurotransmitters and coreleased by the major ascending noradrenergic, serotoninergic, histaminergic, and cholinergic systems. The authors recently reported the existence of functionally significant heteromers of the MOR and galanin 1 receptor (Gal1R) in the ventral tegmental area that could explain these galanin-opioid antagonistic interactions.
The present study sought to answer 2 main questions that arose from our study of MOR-Gal1R heteromers: (a) What are the mechanisms involved in the interactions between galanin and opioid ligands within the MOR-Gal1R heteromer? and (b) Do these interactions also involve morphine and synthetic opioids, such as methadone or fentanyl, differentially?
Results from nanoScan PET/CT
For the animal experiments, the authors have used a nanoScan PET/CT, which provided high resolution and sensitivity to follow the [18F]FDG uptake in the selected brain regions, and find significant differences after using the below mentioned drugs in rats.
The timeline of the experiment was slightly different from the usual PET/CT scans, as it involved two acquisitions: for the baseline scan, saline was injected i.p. (1 ml/kg) into the rats, and after 30 mins, [18F]FDG tracer was applied (i.p. as well). After 30 mins post-injection time, conventional 20 mins long PET/CT acquisitions was performed. After 2 days, the second part was coming, but instead of saline, morphine (1 mg/kg) or methadone (1 mg/kg) was injected i.p. After accessing [18F]FDG, the second PET/CT was conducted as before. For the reconstruction, Teratomo 3D engine was used with attenuation and scatter corrections, with a 0.4 mm resolution.
Figure 4. shows the main results from the PET/CT acquisitions: A. The timeline of the experiment (explained above). B. [18F]FDG uptake after administration of saline (baseline, n = 14), morphine (1 mg/kg, n = 7), or methadone (1 mg/kg, n = 7). Coronal and sagittal images (1.5 mm anterior to bregma and 1.4 mm lateral from the midline, respectively) show the average SUVR calculated using the whole brain as a reference region. C. Voxel-based parametric mapping analyses revealed significantly decreased metabolic activity from baseline values in a basal forebrain region that included the nucleus accumbens and its projecting areas after morphine, but not methadone, treatment. Statistical parametric maps of significant decreases of [18F]FDG uptake (P < 0.05, paired t test). D. and E. VOI analyses of the frontal cortex (FCx), dorsal striatum (DS), and basal forebrain (BF) region, showing a significant differential pattern of [18F]FDG uptake after administration of morphine (D) or methadone (E).
- The authors found significant pharmacodynamic difference between morphine and methadone that is determined entirely by heteromerization of MORs with Gal1Rs, rendering a profound decrease in the potency of methadone. This finding was explained by the weaker proficiency of methadone in activating the dopaminergic system as compared with morphine and predicted a dissociation of the therapeutic and euphoric effects of methadone, which was corroborated by a significantly lower incidence of self-reports of feeling “high” in methadone-medicated patients.
- These results suggest that μ-opioid–Gal1R heteromers mediate the dopaminergic effects of opioids. The results further suggest a lower addictive liability of some opioids, such as methadone, due to their selective low potency for the μ-opioid–Gal1R heteromer.

Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics
Oskar Vilhelmsson Timmermand1, Jörgen Elgqvist2, Kai A. Beattie3, Anders Örbom1, Erik Larsson4, Sophie E. Eriksson1, Daniel L.J. Thorek5, Bradley J. Beattie6, Thuy A. Tran7,8, David Ulmert1,3,9, Sven-Erik Strand1,4
1Division of Oncology and Pathology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
3Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
4Division of Medical Radiation Physics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
5Department of Radiology, Washington University School of Medicine, Saint Louis, MO, 63108, USA
6Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
7Department of Radiopharmacy, Karolinska University Hospital, Stockholm, Sweden
8Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
9Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at University of California, Los Angeles (UCLA), CA, USA
https://doi.org/10.7150/thno.31179
Summary
Particular metastatic prostate cancers can be treated well with androgen ablating drugs, however resistance is probably developing, and with the increased expression of the Androgen Receptor (AR), the tumor may growths back.
Human Kallikrein 2, which is a downstream molecule of AR pathway, can be a potential target, and the authors have used an antibody (hu11B6) against it. They assessed the efficacy of hu11B6 labeled with a low LET beta-emitter, Lutetium-177 (177Lu) and investigated whether similar tumor killing and AR-enhancement is produced. Moreover, single-photon emission computed tomography (SPECT) imaging of 177Lu is quantitatively accurate and can be used to perform treatment planning. [177Lu]hu11B6 therefore has significant potential as a theranostic agent.
Results from nanoSPECT/CT Plus
For the SPECT/CT studies, the authors have used a nanoSPECT/CT Plus, which is a precise option to follow the biodistribution of 177Luhu11B6, and follow the tumor size in mice with good resolution. Subcutaneous PCa xenografts (LNCaP s.c.) were grown in male mice. Biokinetics at 4-336 h post injection and uptake as a function of the amount of hu11B6 injected at 72 h were studied. Over a 30 to 120-day treatment period the therapeutic efficacy of different activities of [177Lu]hu11B6 were assessed by SPECT/CT imaging besides other options.
Performing the acquisitions, a multipinhole mouse collimator was used and with energy windows of 20% centered over the 56-, 113-, and 208-keV energy peaks of 177Lu. Acquisition time was about 40 min. With the help of the CT images as an anatomical reference, regions of interest (ROI), where drawn for tumor, submandibular glands, liver and heart.
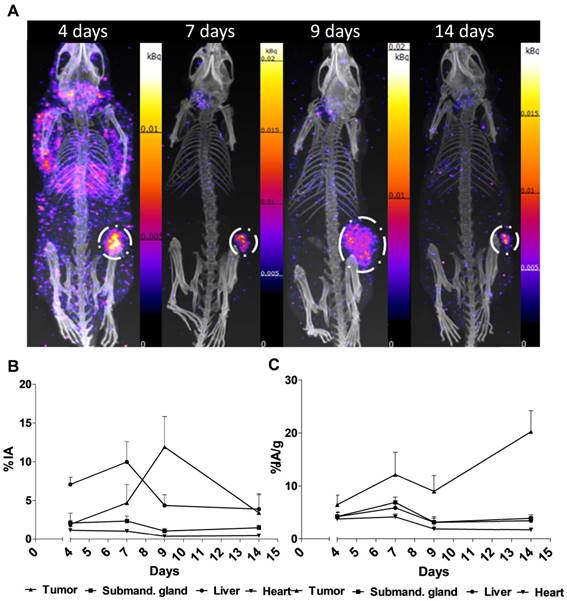
Figure 2. shows the main results from the SPECT/CT acquisitions: A. Representative maximum intensity projections of SPECT/CT of mice at 4, 7, 9 and 14 days p.i. of 177Lu-hu11B6. B. Biokinetics as percent injected activity per gram of tissue (%IA/g) of therapeutic amounts of 177Lu-hu11B6 (20-30 µg) quantified from SPECT data. Quantified data from SPECT/CT imaging of 177Lu-hu11B6 for tumor, submandibular gland, liver and heart. C. Quantified %IA data from SPECT/CT imaging of 177Lu-hu11B6.
- The results suggest tumor accumulation of [177Lu]hu11B6 peaked at 168 h with a specific uptake of 29 ± 9.1 percent injected activity per gram (%IA/g) and low accumulation in normal organs except in the submandibular gland (15 ± 4.5 %IA/g), attributed to a cross-reaction with mice kallikreins in this organ, was seen. However, SPECT imaging with therapeutic amounts of [177Lu]hu11B6 revealed no peak in tumor accumulation at 7 d, probably due to cellular retention of 177Lu and decreasing tumor volumes.
- This study shows that hu11B6 labeled with the low LET beta-emitting radionuclide 177Lu can deliver therapeutic absorbed doses to prostate cancer xenografts with transient hematological side-effects.
18F-FDG-PET Detects Drastic Changes in Brain Metabolism in the Tg4–42 Model of Alzheimer’s Disease
Caroline Bouter1, Philipp Henniges2, Timon N. Franke2, Caroline Irwin2, Carsten Oliver Sahlmann1, Marius E. Sichler2, Nicola Beindorff3, Thomas A. Bayer2 and Yvonne Bouter2
1Department of Nuclear Medicine, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
2Division of Molecular Psychiatry, Department of Psychiatry and Psychotherapy, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
3Berlin Experimental Radionuclide Imaging Center (BERIC), Charité—University Medicine Berlin, Berlin, Germany
https://doi.org/10.3389/fnagi.2018.00425
Summary
The authors have used one of the latest mouse Alzheimer’s Disease (AD) models (Tg4-42 transgenic mutation, which overexpresses the Ab4-42 peptide, which is truncated on the N-terminal region, causing neurotoxicity and Ab aggregation, which are similar to AD). As the AD patients show altered glucose metabolism, the authors chose to follow it with the most common radiopharmaceutical used in PET, namely 18F-FDG, and how it can be used together with the MRI as an early biomarker for AD.
They have found that Tg4-42 mice show a reduction of glucose-metabolism, which correlates with their age, and the decreased 18F-FDG uptake can be shown in early age (3 months).
Results from nanoScan PET/MRI 1T
For the PET/MRI studies, the authors have used a nanoScan PET/MRI 1T, which could provide a fast measurement method together with good statistics. Young (3-4 months) and aged (7-8 months) Tg4-42 (n=7, female) and aged WT C57Bl/6J (7-8 months, n=5, female) control mice were used in this study. The authors have followed the standard 18F-FDG PET/MRI protocol, as the mice were fasted overnight, and 9-21 MBq activity was injected into the tail vein, with a 45 minute long uptake period.
The PET scans were performed for 20 minutes , which was followed by a Tera-Tomo 3D reconstruction method with a 0.3 mm3. For the MRI, the authors have used GRE sequence as a material map for attenuation and scatter correction in the PET reconstruction, and as a brain atlas. The analysis was performed with PMOD software.
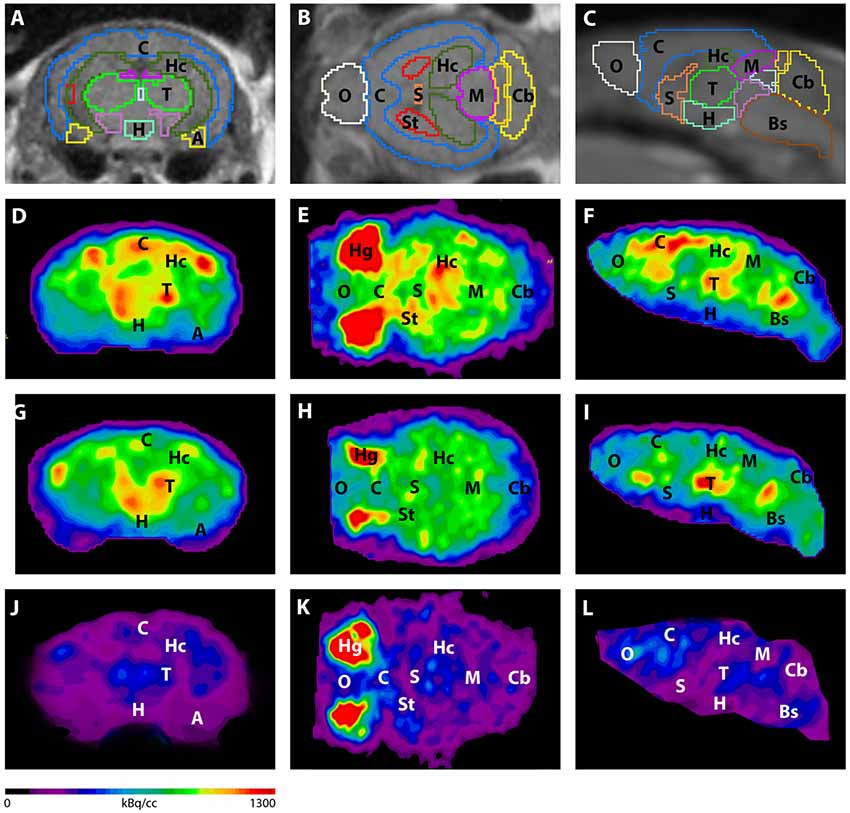
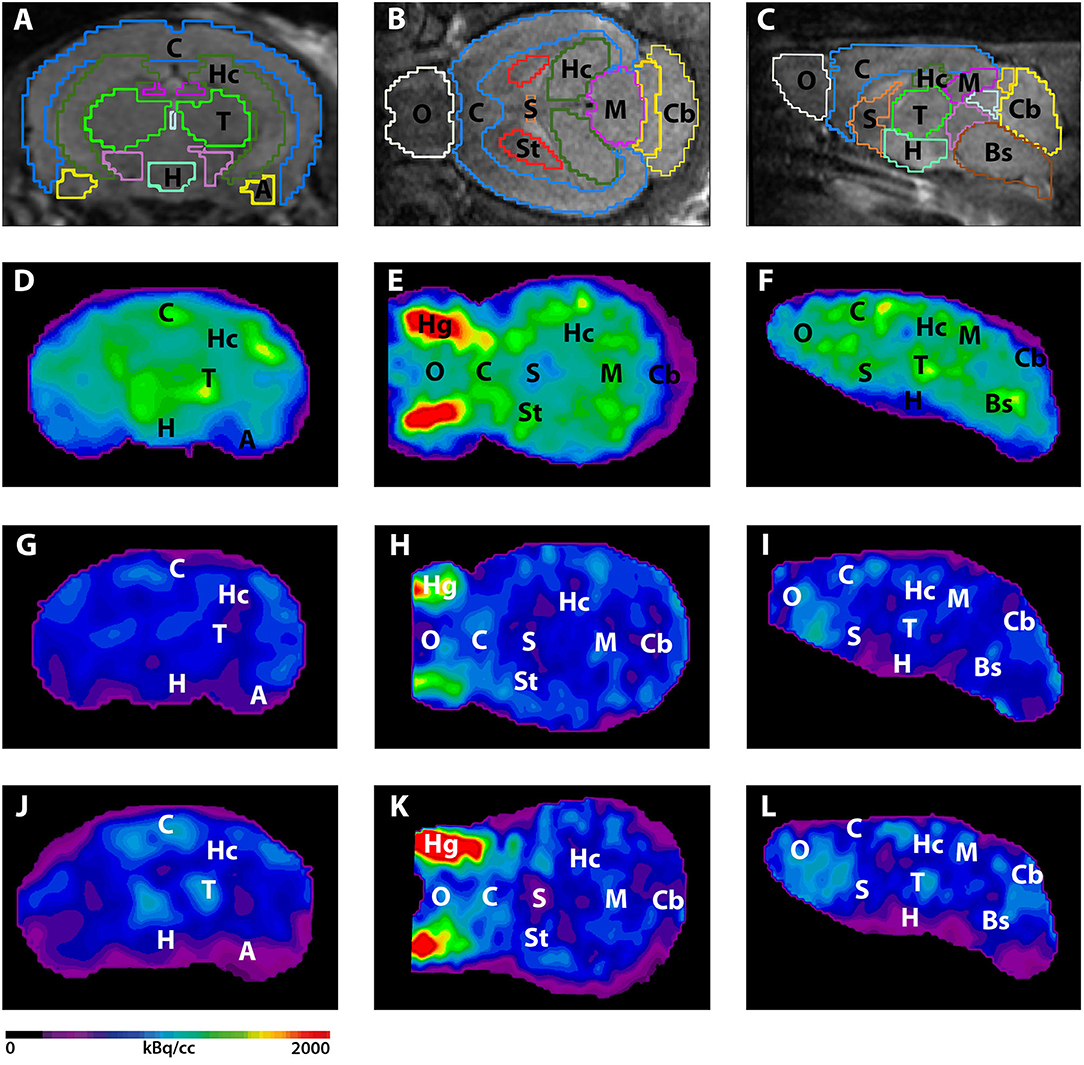
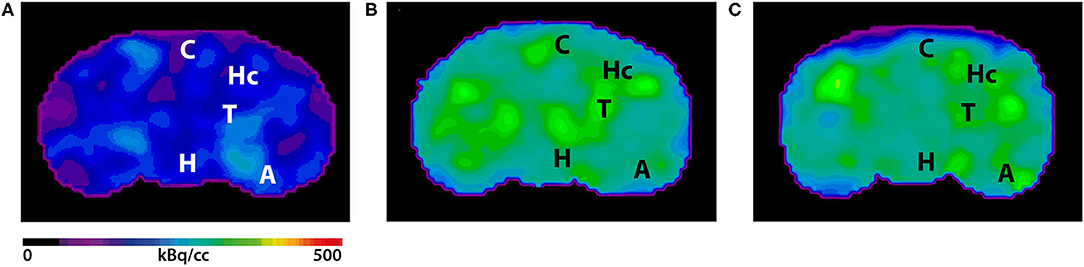
Figure 3. shows the main results from the PET/MRI acquisitions: a-c) MRI images were matched with predefined brain regions; axial, coronal and sagittal view. d-f) 18F-FDG-PET images of a WT mouse. g-i) 18F-FDG-PET images of a young Tg4–42 mouse. j-l) 18F-FDG-PET images of an aged Tg4–42 mouse. A, Amygdala; Bs, Brain Stem; C, Cortex; Cb, Cerebellum; H, Hypothalamus; Hc, Hippocampus; Hg, Harderian glands; M, Midbrain; O, Olfactory Bulb; S, Septum/Basal Forebrain; St, Striatum; T, Thalamus.
- These results suggest that 18F-FDG uptake was distinctly lower in aged Tg4–42 mice compared to WT mice. In young Tg4–42 mice 18F-FDG the uptake did not show significant differences in whole brain uptake but it was reduced in the hippocampus, forebrain, hypothalamus, amygdala and midbrain.
- The study showed that Tg4-42 can be a useful AD model to monitor the effects of various therapeutic strategies in vivo using 18F-FDG uptake as a therapeutic readout.