Validation of Image Qualities of a Novel Four-Mice Bed PET System as an Oncological and Neurological Analysis Tool
Kyung Jun Kang1, Se Jong Oh1, Kyung Rok Nam1, Heesu Ahn1,2, Ji-Ae Park1,2, Kyo Chul Lee1, Jae Yong Choi1,2
1Division of Applied RI, Korea Institute of Radiological and Medical Sciences, Seoul 01812, Korea
2Radiological and Medico-Oncological Sciences, University of science and technology (UST), Seoul 01812, Korea
https://doi.org/10.3390/jimaging7030043
Summary
Molecular imaging is used for the diagnosis of oncological, neurological, and cardiovascular diseases. Positron emission tomography (PET) is a molecular imaging technique that enables quantitative measurements of biochemical parameters, such as metabolic changes and concentrations of neurotransmitters. The demand for preclinical research is increasing worldwide, as it can predict clinical trials and advance our understanding of the pathophysiological mechanisms underlying a specific disease. For this purpose, researchers use micro-PET (µPET), which is a small-animal dedicated system. Most µPET vendors provide a single bed, thereby allowing imaging of only a single animal at a time. Large-scale research involving many objects thus requires tremendous time and use of radioactivity.
Recently, Mediso developed a multi-bed system dedicated to the nanoScan scanners with the contribution of Tim Witney and his team at UCL and KCL, where the initial validity for research has been investigated. Greenwood et al. (https://doi.org/10.2967/jnumed.119.228692) tested the four-bed mouse system using 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) in phantom and normal mice and reported a quantitative accuracy similar to that of a single-bed. To date, however, few studies have focused on the validity of oncological and neurological PET imaging of the four-mice bed system.
This study aimed to evaluate the image qualities of oncological and neurological PET imaging using a novel four-mice bed system.
Results from the nanoScan PET/CT
For the small animal imaging, the authors have used a nanoScan PET/CT, to see the differences using the single-bed vs. multi-bed scans regarding the image quality in oncological and neurological studies. The scanner has a peak absolute system sensitivity of >9% in the 400–600 keV energy window, an axial field of view of 28 cm, a transaxial field of view of 35–120 mm, and a transaxial intrinsic resolution of 0.7 mm at 1 cm off-center.
For the phantom studies, following the guidelines of NEMA NU-4 2008, four phantoms were filled with 3.7 MBq of 18F-FDG in 10 mL of saline and decay-corrected to the start of acquisition. On the first day, four phantoms were imaged in the multi-bed system, and one day later a single phantom was imaged using a single-bed system for comparison. Static PET acquisition was performed using the nanoScan PET/CT over 20 min, followed by CT (480 projections; 50 kVp tube voltage; 630 µA; 300 ms exposure time; 1:4 binning). Whole-body TeraTomo 3D reconstruction with four iterations and six subsets was performed (1–5 coincidence mode) using an isotropic voxel size of 0.4 mm3.
In all the preclinical experiments, four animals were scanned in the multi-bed system, and then, single PET images were acquired using the single-bed system 2 days later. For the oncological study, prior to imaging, all mice fasted overnight. The mice were anesthetized with 2.5% isoflurane in oxygen, and 18F-FDG (8.8 ± 0.9 MBq/200 µL) was administrated into the tail vein. Based on previous studies, 18F-FDG images were acquired after 40–60 min. For the brain study, an 18F-FPEB PET tracer (8.7 ± 0.7 MBq/200 µL) was prepared. 18F-FPEB PET images were acquired 30–50 min after injection because the radiotracer initially shows specific-to-nonspecific binding values that reach a plateau after 30 min. Images were reconstructed using TeraTomo 3D algorithm with four iterations. For attenuation correction, CT imaging was performed immediately after PET using 50 kVp of X-ray voltage with 0.16 mAs.
Figure 3. shows the main results from the oncological study, with representative PET/CT images of tumor-bearing mice after 18F-FDG injection in single- and multi-bed systems (A). The plane was chosen to best reflect the tracer uptake characteristics of each group. Comparison of tumor uptake values in single- and multi-bed systems based on tumor size (B,C), **** p < 0.0001, n.s.= statistically nonsignificant. PET/CT, positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value.
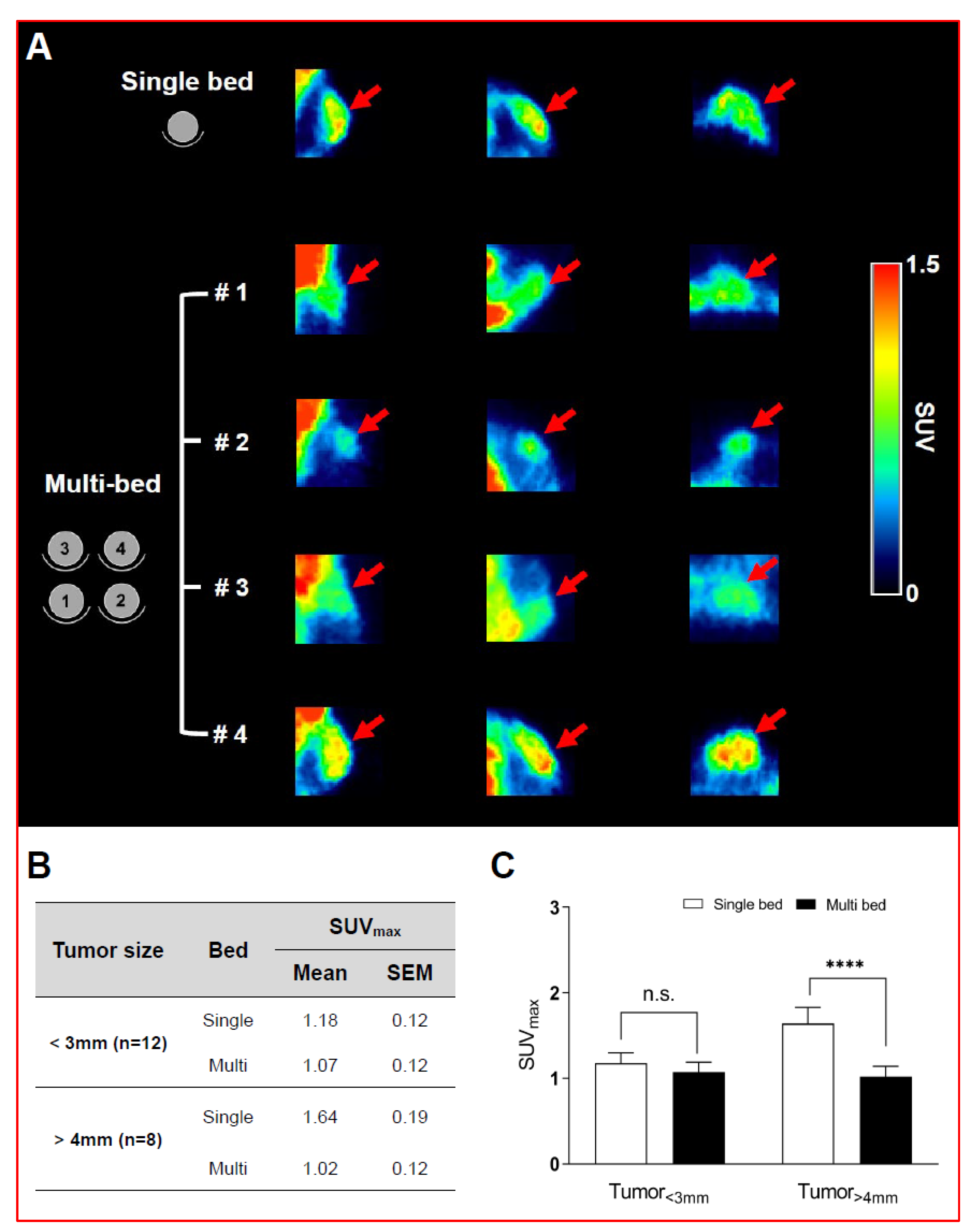
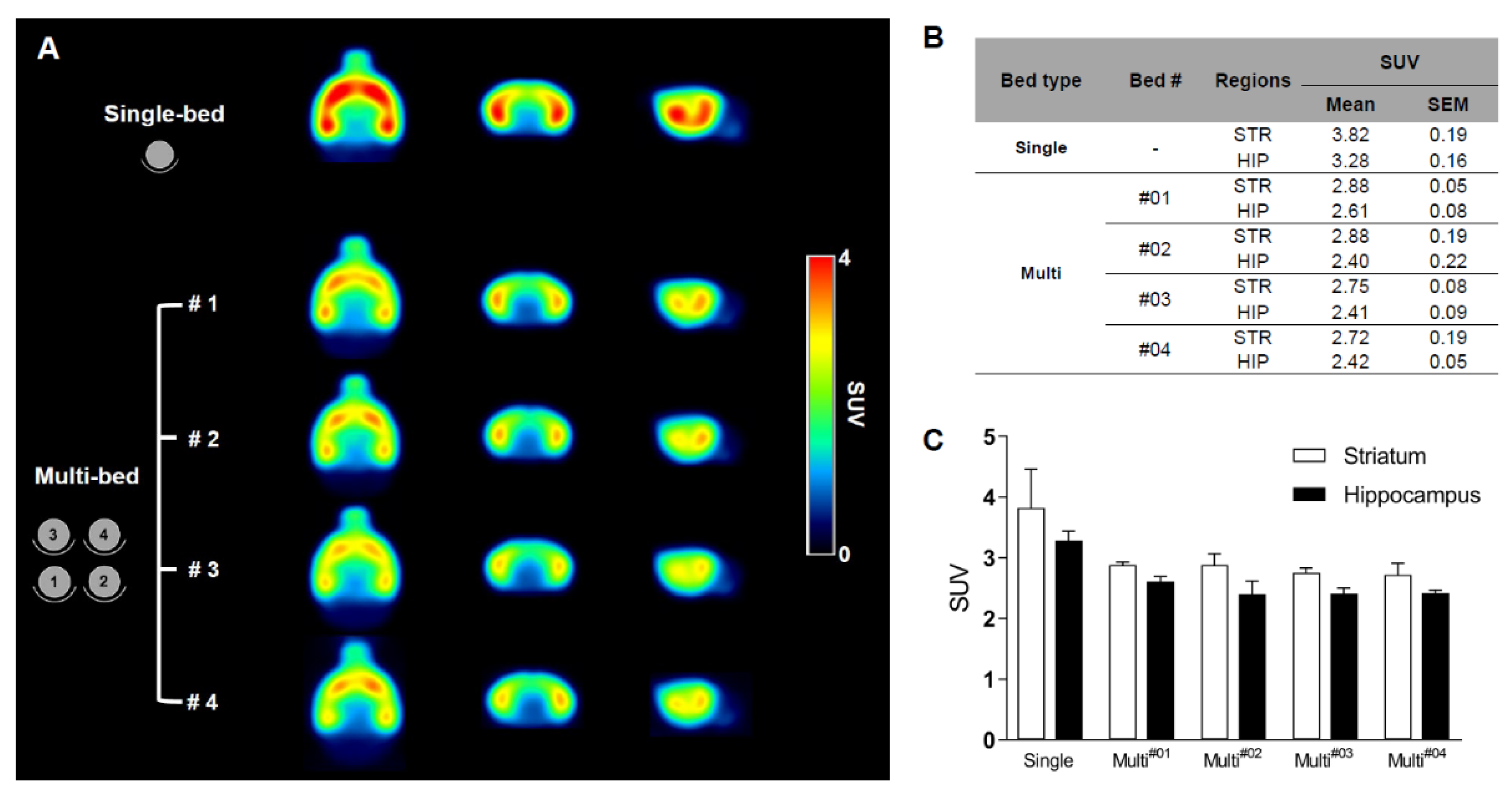
Figure 4. shows the results from the neurological study. Summed 18F-FPEB brain PET images of the single-bed (n = 12) and each bed of the multi-bed system (A, n = 3 in each bed). Comparison of regional SUV values of single- and multi-bed systems (B,C). STR, striatum; HIP, hippocampus; SUV, standardized uptake value.
- PET images acquired with the multi-bed system showed a lower estimation of radiation uptake compared to that of the single-bed system when images were acquired for small lesions. However, phantom studies showed that there were no differences between the single- and multi-bed systems for volumes > 4 mm and that multi-bed systems can be applied in oncological and neurological studies.
- The multi-bed system may improve the efficacy of preclinical research by saving the time taken to acquire PET images.