Innovation Delivered
The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule
Maryam Oroujeni1, Tianqi Xu1, Katherine Gagnon2,3, Sara S. Rinne3, Jan Weis4, Javad Garousi1, Ken G. Andersson5, John Löfblom5, Anna Orlova3,6, Vladimir Tolmachev1,6
Effects of administration route on uptake kinetics of 18F‑sodium fluoride positron emission tomography in mice
Zaniah N. Gonzalez‑Galofre1,2, Carlos J. Alcaide‑Corral1,2 & Adriana A. S. Tavares1,2
1British Heart Foundation/University of Edinburgh Centre for Cardiovascular Science
2Edinburgh Imaging, University of Edinburgh, Little France Campus, Edinburgh
3British Heart Foundation/University of Edinburgh Centre for Cardiovascular Science
https://doi.org/10.1038/s41598-021-85073-0
Summary
18F-sodium fluoride (18F-NaF) is a positron emission tomography (PET) radiotracer widely used in skeletal imaging and has also been proposed as a biomarker of active calcification in atherosclerosis. Like most PET radiotracers, 18F-NaF is typically administered intravenously.
However, in small animal research intravenous administrations can be challenging, because partial paravenous injection is common due to the small calibre of the superficial tail veins and repeat administrations via tail veins can lead to tissue injury therefore limiting the total number of longitudinal scanning points. In this paper, the feasibility of using intra-peritoneal route of injection of 18F-NaF to study calcification in mice was studied by looking at the kinetic and uptake profiles of normal soft tissues and bones versus intravascular injections.
In soft tissue, the 18F-NaF perfusion phase changes depending on the type on injection route, whereas the uptake phase is similar regardless of the administration route. In bone tissue standardised uptake value (SUV), standardised uptake value ratio (SUVr) and uptake constant (Ki) measures were not significantly different between the three administration routes.
Authors have concluded that, intra-peritoneal injection is a valid and practical alternative to the intra-vascular injections in small-animal 18F-NaF PET imaging and provides equivalent pharmacokinetic data. Furthermore, their data show that CT outcomes report on sites of stablished calcification whereas PET measures sites of higher complexity and active calcification.
Results from nanoScan PET/CT
Mice received an intra-vascular or intra-peritoneal injection of 18F-NaF as follows: intra-arterial administration (8.68 ± 3.94 MBq, mean ± SD, n = 7) via femoral artery; intra-venous administration via femoral vein (7.43 ± 4.69 MBq, mean ± SD, n = 6); and intra-peritoneal administration (7.85 ± 2.18 MBq, mean ± SD, n = 6). Immediately post-radiotracer administration, a 60 min whole-body emission scan was obtained. PET data was reconstructed into 6 × 30 s, 3 × 60 s, 2 × 120 s, 10 × 300 s frames using Mediso’s iterative Tera-Tomo 3D reconstruction algorithm and the following settings: 4 iterations, 6 subsets, full detector model, low regularisation, spike filter on, voxel size 0.4 mm and 400–600 keV energy window.

Figure 1. Representative examples of biodistribution of 18F-sodium fluoride (NaF) after femoral artery, femoral vein and intra peritoneal injections presented as average sagittal section images between 45 and 60 min post-injection (A). Mean time-activity curves of the vena cava (B), heart (C), lungs (D), liver (E) and kidneys (F) after femoral, artery, femoral vein or intraperitoneal (IP) injection. Error bars represent one SEM. SUV standardised uptake value.
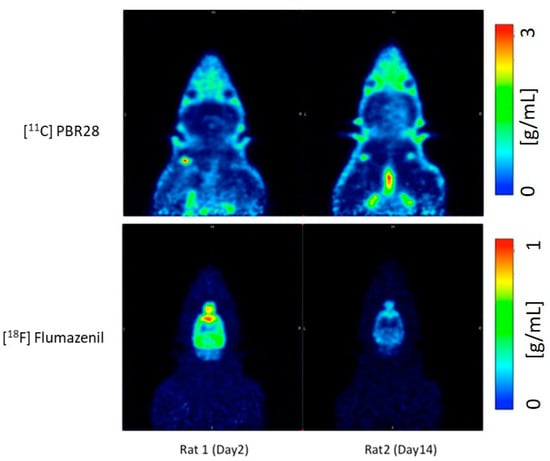
An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with 11C-PBR28 and 18F-flumazenil PET Imaging
Krishna Kanta Ghosh1, Parasuraman Padmanabhan1,2, Chang-Tong Yang1,3,4, Zhimin Wang1, Mathangi Palanivel1 , Kian Chye Ng5, Jia Lu5, Jan Carlstedt-Duke6, Christer Halldin1,7 and Balázs Gulyás1,2,7
1 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore
2 Cognitive Neuroimaging Centre, Nanyang Technological University, Singapore
3 Department of Nuclear Medicine and Molecular Imaging, Radiological Sciences Division, Singapore General Hospital, Singapore
4 Duke-NUS Medical School, Singapore
5 DSO National Laboratories (Kent Ridge), Singapore;
6 President’s Office, Nanyang Technological University, Singapore;
7 Department of Clinical Neuroscience, Karolinska Institute, Sweden
https://doi.org/10.3390/ijms22020951
Summary
Traumatic brain injury (TBI) modelled by lateral fluid percussion-induction (LFPI) in rats is a widely used experimental rodent model to explore and understand the underlying cellular and molecular alterations in the brain caused by TBI in humans. The present study aims to investigate whether the two adioligands, 11C-PBR28 and 18F-flumazenil, are able to accurately quantify in vivo molecular-cellular changes in a rodent TBI-model for two different biochemical targets of the processes. As 11C-PBR28 is a radioligand of the 18 kD translocator protein (TSPO), the up-regulation of which is coupled to the level of neuroinflammation in the brain, and 18F-flumazenil is a radioligand for GABAA-benzodiazepine receptors, whose level capable of indicating at a high precision the neuronal loss that ensues in various brain disorders and injuries, the use of the two radioligands may reveal two critical features of TBI. An up-regulation in the 11C-PBR28 uptake triggered by the LFP in the injured (right) hemisphere was noted on day 14, while the uptake of 18F-flumazenil was down-regulated on day 14. When comparing the left (contralateral) and right (LFPI) hemispheres, the differences between the two in neuroinflammation were obvious. In vitro immunohistochemical analyses on the corpus callosum and hippocampal sections of the cerebrum were done to validate the results obtained in the PET imaging Results demonstrate a potential way to measure the molecular alterations in a rodent-based TBI model using PET imaging with 11C-PBR28 and 18F-flumazenil. These radioligands are promising options that can be eventually used in exploring the complex in vivo pharmacokinetics and delivery mechanisms of nanoparticles in TBI treatment.
Results from nanoScan PET/MRI
According to a standard LFP procedure, a combination of focal and diffuse injury was inflicted on the cerebral cortex and hippocampus of the right hemisphere of rats to create a TBI model for the study. The left hemisphere served as an internal control for the study. Following the LFP procedure on the brain’s right hemisphere of 12 Sprague Dawley rats, day 2 post operation 3D dynamic PET scans were performed using the nanoScan PET/MRI scanner. After injecting approximately 30±4MBq 11C-PBR28 or 18±4MBq 18F-flumazenil to the tail vein, dynamic 63min PET scan was performed with 11C-PBR28. The second PET radioligand 18F-flumazenil was injected 80min later, and the animals were scanned for a duration of 90min. The detailed time frames for the respective scan protocols were as follows: 8x15s, 4x30s, 2x1min, 2x2min, 4x5min, 3x10min for 11C-PBR28; 8x15s, 4x30s, 2x1min, 2x2min, 4x5min and 6x10min for 18F-flumazenil.
Results show:
- Compared to day 2 post-op, there is an increase in the uptake of 11C-PBR28 on day 14 due to the LFP in the right hemisphere (injured)
- 18F-flumazenil uptake was down-regulated on day 14, compared to day 2
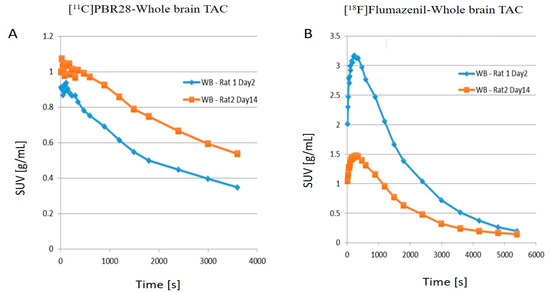
- The time activity curves (TACs) of the whole brain also clearly demonstrate that there was a higher 11C-PBR28 and lower 18F-flumazenil uptake in day 14 as compared to day 2
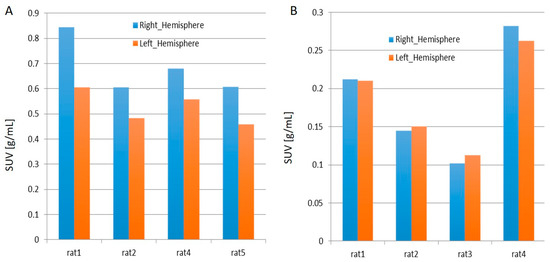
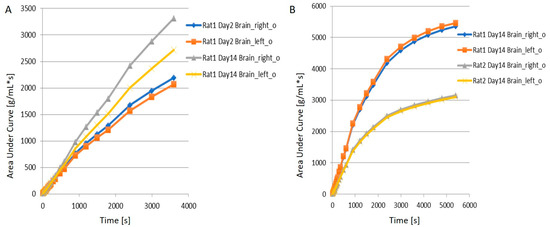
- When juxtaposing the right and left hemispheres using an area-under-the-curve (AUC) measure, the discrepancies between the two hemispheres for the 11C-PBR28 radiotracer were apparent. This is an indication that local increases in neuroinflammation due to the physical impact can be observed in the LFPI TBI rodent model. On the other hand, while 18F-flumazenil uptake is slightly higher in the left hemisphere, the lack of marked changes between the two hemispheres may reflect either the lack of neuron density alterations or the inappropriateness of the radioligand in indicating neuron density changes. This is the first LFPI TBI rat study to evaluate neuroinflammation and loss of neuronal density using two radioligands subsequently on the same day.
Myocardial perfusion recovery induced by an a-calcitonin gene-related peptide analogue
Simon Bentsen, MD1, Anette Sams, PhD2, Philip Hasbak, MD, DMSc1, Lars Edvinsson, MD, PhD, DMSc2, Andreas Kjaer, MD, PhD, DMSc1, Rasmus S. Ripa, DMSc1
1Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark
2Department of Clinical Experimental Research, Glostrup Research Institute, Glostrup University Hospital, Glostrup, Denmark
https://doi.org/10.1007/s12350-021-02678-8
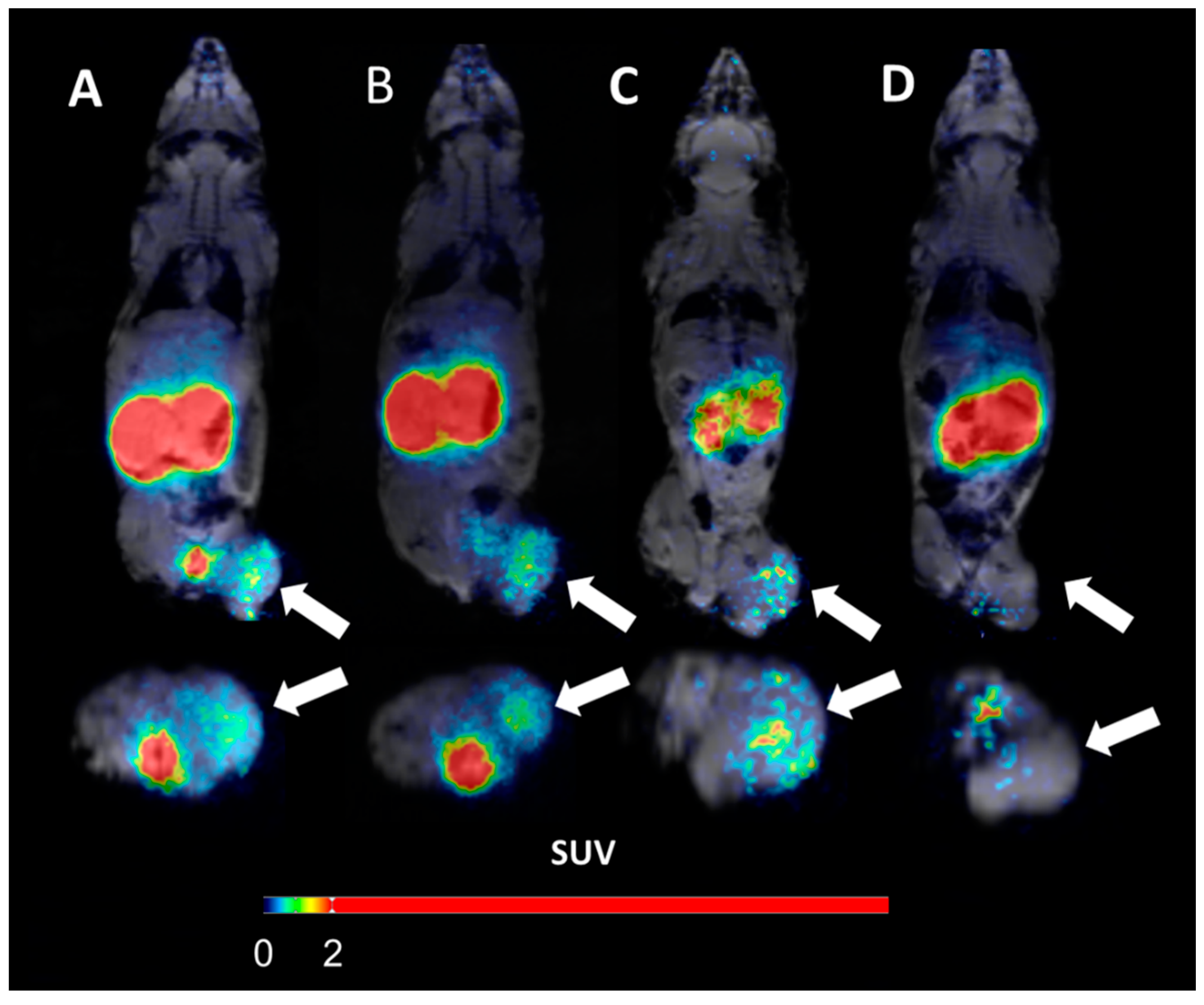
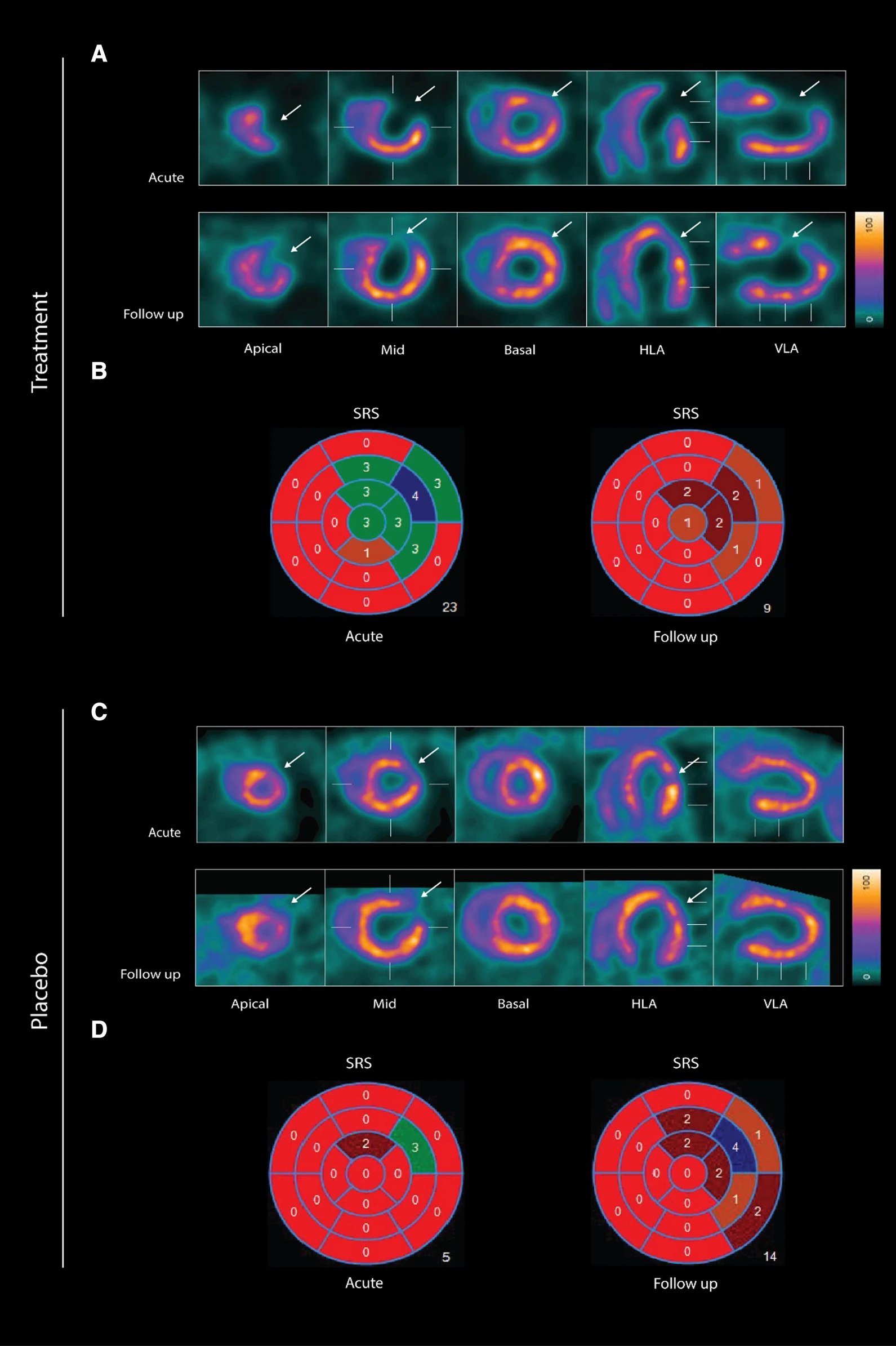
wo representative examples of rats with chronic LAD occlusion from the SPECT/CT scans. 4DM software was used to analyze [99mTc]Tc-sestamibi SPECT/CT. Top panels (A and B) show a SAX-treated rat. Bottom panels (C and D) show a placebo-treated rat.(A) Large perfusion defect in the anterior and apical wall in the acute scan, with a smaller perfusion defect at follow-up scan after SAX treatment (white arrow).(B) The perfusion defects from panel A in a 17-segment polar map.(C) Medium perfusion defect in the anterior wall at the acute scan, and a more severe perfusion defect at follow-up after placebo treatment (red arrow).(D) The perfusion defects from panel C in a 17-segment polar map. HLA horizontal long axis, VLA vertical long axis, SRS summed rest score.
- The results show that an analogue of CGRP that induces both coronary and peripheral vasodilation significantly improves myocardial perfusion recovery after experimental myocardial infarction in rats. There was no significant difference in overall survival between the two groups suggesting that SAX does not have a damaging effect on the animals or the myocardium.
- In conclusion, the CGRP analogue, SAX, seems to have a cardioprotective effect on a rat model of myocardial infarction, by improving the perfusion recovery after a chronic occlusion of the coronary artery.
One Size Fits All? Not in In Vivo Modeling of Tuberculosis Chemotherapeutics
Hee-Jeong Yang1, Decheng Wang2 , 3 , Xin Wen2 , 3 ,Danielle M. Weiner1 , 4 and Laura E. Via1 , 4 , 5 , *
1 Tuberculosis Research Section, Laboratory of Clinical Immunology and Microbiology, Division of Intramural Research (DIR), National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH), Bethesda, MD, United States,
2 Medical College, China Three Gorges University, Yichang, China,
3 Institute of Infection and Inflammation, China Three Gorges University, Yichang, China,
4 Tuberculosis Imaging Program, DIR, NIAID, NIH, Bethesda, MD, United States,
5 Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa
doi: 10.3389/fcimb.2021.613149
Abstract
Tuberculosis (TB) remains a global health problem despite almost universal efforts to provide patients with highly effective chemotherapy, in part, because many infected individuals are not diagnosed and treated, others do not complete treatment, and a small proportion harbor Mycobacterium tuberculosis (Mtb) strains that have become resistant to drugs in the standard regimen. Development and approval of new drugs for TB have accelerated in the last 10 years, but more drugs are needed due to both Mtb’s development of resistance and the desire to shorten therapy to 4 months or less. The drug development process needs predictive animal models that recapitulate the complex pathology and bacterial burden distribution of human disease. The human host response to pulmonary infection with Mtb is granulomatous inflammation usually resulting in contained lesions and limited bacterial replication. In those who develop progressive or active disease, regions of necrosis and cavitation can develop leading to lasting lung damage and possible death. This review describes the major vertebrate animal models used in evaluating compound activity against Mtb and the disease presentation that develops. Each of the models, including the zebrafish, various mice, guinea pigs, rabbits, and non-human primates provides data on number of Mtb bacteria and pathology resolution. The models where individual lesions can be dissected from the tissue or sampled can also provide data on lesion-specific bacterial loads and lesion-specific drug concentrations. With the inclusion of medical imaging, a compound’s effect on resolution of pathology within individual lesions and animals can also be determined over time. Incorporation of measurement of drug exposure and drug distribution within animals and their tissues is important for choosing the best compounds to push toward the clinic and to the development of better regimens. We review the practical aspects of each model and the advantages and limitations of each in order to promote choosing a rational combination of them for a compound’s development.
Results from MultiScan LFER PET/CT
Tuberculosis Imaging group under the direction of Laura E. Via is using 2 MultiScan LFER PET/CT scanner for studying tuberculosis on Rabbit, Common Marmoset and Rhesus Macaque at National Institute of Allergy and Infectious Diseases.
In this substantial comprehensive review, the researchers also compared these 3 animal models in respect of PET/CT imaging:
- In longitudinal studies that combined PET/CT imaging, using FDG injected IV as a probe for metabolic activity, FDG concentrated in metabolically active regions within the Mtb HN878 lesions as they developed, but as necrosis progressed, the acellular centers of granulomas and cavities accumulated less FDG due to the lack of live cells (Figure 3A)
- NHP models are amenable to most diagnostic and therapeutic methods used in clinical studies, such as medical imaging (Figures 3B, C), serial blood sampling, and bronchoalveolar lavage (BAL) sampling for pharmacokinetic monitoring within an individual (Lewinsohn et al., 2006; Lin et al., 2013; Via et al., 2013). As serial imaging is feasible, methods to follow both individual lesion development and regression PI have been developed

Figure 3. FDG PET/CTs of a rabbit (A), marmoset (B), and rhesus macaque (C) with cavitary disease. The animals were infected for 69 to 90 days with M. tuberculosis at the time of imaging. Cavities (blue arrows) have been partially emptied of their necrotic contents and filled with air indicated by a darker central region in the lesions (lower density) surrounded by lighter walls (higher density). The scales show the range of CT Hounsfield units from higher to low density in shades of gray (+400 to −1000) on the left and FDG uptake in PET standard uptake units/body weight from high to low uptake in bright yellow to red to black (14 to 0) on the right. The width of the animal’s midsection is indicated with a bar and label to highlight the difference in size of the three animals.
Para-chloro-2-[18F]fluoroethyl-etomidate: A promising new PET radiotracer for adrenocortical imaging
Isabella Silins1, Anders Sundin1, Patrik Nordeman2, Mahabuba Jahan2, Sergio Estrada2, Azita Monazzam3, Mark Lubberink1, Franklin Aigbirhio4, Per Hellman1, Gunnar Antoni2
1 Department of Surgical Sciences, Uppsala University
2 Medicinal Chemistry and Uppsala University
3 Medical Sciences at Uppsala University
4 Wolfson Brain Imaging Centre, University of Cambridge
https://www.medsci.org/v18p2187.htm
Summary
11C-Metomidate (11C-MTO) was developed as a PET radiotracer for adrenocortical tumours and has also been suggested for imaging in primary aldosteronism (PA). However, the use of 11C-MTO is somewhat hampered by considerable accumulation in the liver, which, because of its close proximity to the right adrenal gland, may obscure adrenal pathology and make PET measurements unreliable. Moreover, increased 11C-MTO uptake has been found in various liver lesions, such as adenoma, hepatocellular cancer and focal nodular hyperplasia, with the risk of false positive imaging results. Another disadventage of the tracer is that the clinical availability is restricted because of the short half-life of carbon-11 (T1/2= 20.4 min), which limits its use to PET centres with an in-house cyclotron and radiopharmacy.
The aim of this study was to evaluate the binding properties and in vivo behaviour of the previously published 18F-labeled (halflife 109.5min) etomidate analogue, para-chloro-2-18F-fluoroethyl etomidate; (18F-CETO), as an adrenal PET tracer. Comparative studies were also performed with 11C-MTO and with 18F-FETO, another adrenocortical imaging agent, which has not reached widespread clinical use, partial due to its two-stage radiosynthesis.
Autoradiography on human and cynomolgus monkey tissues show specific, high 18F-CETO uptake in normal adrenal cortex, as well as in human adrenocortical adenomas and adrenocortical carcinomas.
Following in vitro binding kinetic analysis and the evaluation of ex vivo biodistribution, in vivo imaging studies revealed high specificity of 18F-CETO accumulation in the adrenal cortex qualitatively surpassing those of 11C-MTO. Non-specific binding to the liver was significantly lower than that of 11C-MTO. 18F-CETO is a promising new PET tracer for imaging of adrenocortical disease and should be evaluated further in humans.
Results from nanoScan PET/MRI
18F-CETO imaging in rats and mice:
Eight female C57BL/6 mice and two male Sprague Dawley rats were were injected with of 18F-CETO (1.6±1MBq or 4-5MBq, respectively) and the 1h long dynamic PET imaging was started immediately. Four of the mice and one of the rats were co-injected with metomidate (1μmol/kg). The PET examination was followed by an MRI acquisition.
- 18F-CETO accumulated predominantly in the liver and in the adrenal glands, thus obfuscating the view of the adrenal glands in mice (Figure 1A: baseline; B: after blockage with metomidate)
- in contrast in rats the uptake was concentrated mainly in the adrenal glands with 120min p.i. peak adrenal uptake (Figure 2A: baseline; B: after blockage with metomidate)
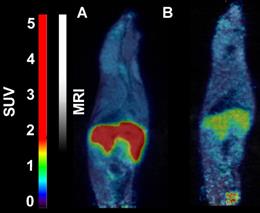
18F-FETO imaging in rats:
Rats were were iv. injected with 4.0-4.8 MBq of 18F-FETO, one of the rats was given metomidate (1μmol/kg) and 1h long PET scan was started immediately.
- 18F-FETO in rats was also concentrated mainly in the adrenal glands. However, it was unable to block the uptake with metomidate, thus, re-evaluation of 18F-FETO has to be discontinued (Figure 2C: baseline; D: after blockage with metomidate)
Bispecific GRPR-Antagonistic Anti-PSMA/GRPR Heterodimer for PET and SPECT Diagnostic Imaging of Prostate Cancer
Bogdan Mitran1, Zohreh Varasteh1,2, Ayman Abouzayed1, Sara S. Rinne1, Emmi Puuvuori1, Maria De Rosa1,3, Mats Larhed1,4, Vladimir Tolmachev5, Anna Orlova1,4, Ulrika Rosenström1
1Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
2Department of Nuclear Medicine, Klinikum rechts der Isar der TUM, 81675 Munich, German
3Drug Discovery Unit, Ri.MED Foundation, 90133 Palermo, Italy
4Science for Life Laboratory, Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
5Department of Immunology, Genetics and Pathology, Uppsala University, 751 23 Uppsala, Sweden
https://doi.org/10.3390/cancers11091371
Whole body SPECT/CT scans of the mice bearing PC3-PIP xenografts injected with 111In-6 (400 kBq, 100 pmol/mouse) were performed using nanoScan SPECT/CT at 1, 3, and 24 h pi. Additionally, three mice were co-injected with either non-labeled PSMA-617 (1.5 nmol), non-labeled NOTA-PEG6-RM26 (1.5 nmol), or a combination of both, and imaged 1 h pi. CT scans were acquired at the following parameters: 50 keV, 670 μA, 480 projections, and 5 min acquisition time. SPECT scans were carried out using 111In energy windows (154 keV–188 keV; 221 keV–270 keV), a 256 × 256 matrix, and a 30 min acquisition time. The CT raw data were reconstructed using Nucline 2.03 Software. SPECT raw data were reconstructed using Tera-Tomo™ 3D SPECT.
PET/CT scans of the mice injected with 68Ga-6 (1.8 MBq, 100 pmol/mouse) were performed using nanoScan PET/MRI 3T at 1 h pi. To evaluate the in vivo specificity, one mouse was co-injected with a combination of non-labeled PSMA-617 (1.5 nmol) and non-labeled NOTA-PEG6-RM26 (1.5 nmol). CT acquisitions were performed as previously described using nanoScan SPECT/CT immediately after PET acquisition employing the same bed position. PET scans were performed for 30 min. PET data were reconstructed into a static image using the Tera-Tomo™ 3D reconstruction engine. CT data were reconstructed using Filter Back Projection. PET and CT files were fused and analyzed using Nucline 2.03 Software.
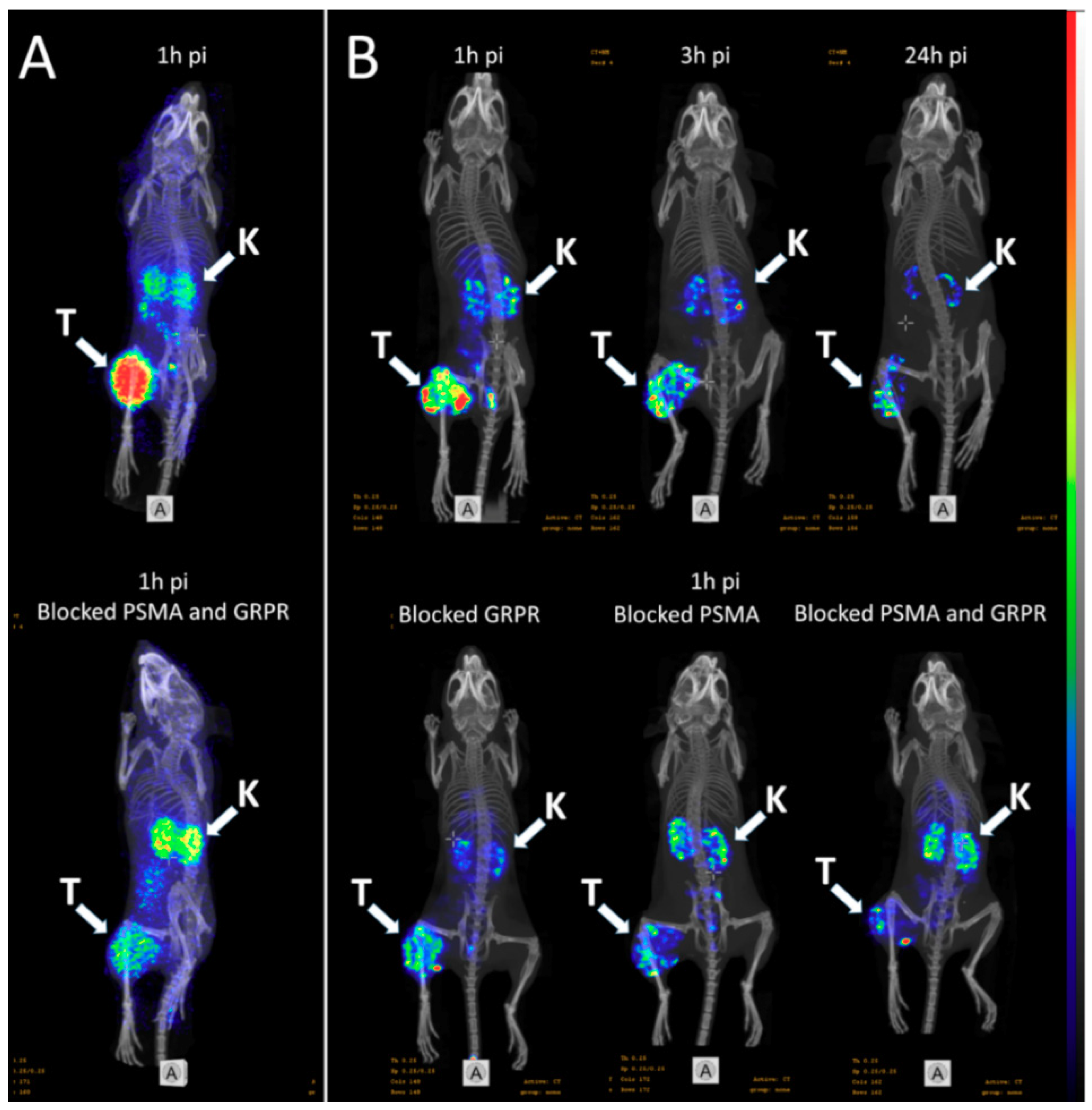
(A) micro positron emission tomography (microPET)/CT and (B) microSPECT/CT MIP images of PC3-PIP-xenografted mice (PSMA positive/GRPR positive) after an iv injection of 68Ga-6 and 111In-6. Blocked animals were co-injected with PSMA-617 for the blocking of PSMA and RM26 for the blocking of GRPR, or both.
- The imaging of PC3-PIP tumors using microPET/CT for 68Ga-6 (1 h pi) and microSPECT/CT for 111In-6 (1, 3, and 24 h pi) confirmed the ex vivo biodistribution data. Tumors were clearly visualized at all time-points, while weak activity accumulation was only observed in the kidneys among healthy organs. In agreement with ex vivo measurements, imaging contrast improved with time up to 3 h pi, despite the rapid washout of activity from tumors. The superior imaging contrast obtained for 111In-6 compared to 68Ga-6 was also in agreement with the biodistribution data. The imaging of mice after the co-injection of excess PSMA- and GRPR-targeting monomers corroborated with the ex vivo biodistribution pattern: partially decreased activity uptake in tumors, but increased activity uptake in kidneys, were exhibited in the case of PSMA blocking.
- MicroPET/CT and microSPECT/CT scans confirmed biodistribution data, suggesting that 68Ga-NOTA-DUPA-RM26 and 111In-NOTA-DUPA-RM26 are suitable candidates for the imaging of GRPR and PSMA expression in PCa shortly after administration.
Sigma-1 Receptor Positron Emission Tomography: A New Molecular Imaging Approach Using (S)-(−)-[18F]Fluspidine in Glioblastoma
Magali Toussaint 1, Winnie Deuther-Conrad 1, Mathias Kranz 1 2 3, Steffen Fischer 1, Friedrich-Alexander Ludwig 1, Tareq A Juratli 4, Marianne Patt 5, Bernhard Wünsch 6, Gabriele Schackert 4, Osama Sabri 5, Peter Brust 1
1 Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Institute of Radiopharmaceutical Cancer Research, Department of Neuroradiopharmaceuticals, Research site Leipzig, 04318 Leipzig, Germany.
2 PET Imaging Center, University Hospital of North Norway (UNN), 9009 Tromsø, Norway.
3 Nuclear Medicine and Radiation Biology Research Group, The Arctic University of Norway, 9009 Tromsø, Norway.
4 Department of Neurosurgery, Technische Universität Dresden (TUD), University Hospital Carl Gustav Carus, 01307 Dresden, Germany.
5 Department of Nuclear Medicine, University Hospital Leipzig, 04318 Leipzig, Germany.
6 Institute of Pharmaceutical and Medicinal Chemistry, University of Münster, 48149 Münster, Germany.
https://doi.org/10.3390/molecules25092170
Summary
Glioblastoma multiforme (GBM) is the most common primary tumors of the central nervous system. The survival rate for patients with GBM is dramatically low compared to patients with other brain tumor types. An important aspect contributing to this poor outcome is the genetic heterogeneity of GBM, which translates into heterogeneous expression patterns of potentially druggable targets. Hence the understanding of how spatiotemporal patterns evolve and change during pathogenesis would help to develop new targeted therapies, and biomarkers for treatment response.
The sigma-1 receptor (sig1R), an endoplasmic reticulum chaperone protein, is involved in signaling pathways assumed to control the proliferation of cancer cells and thus could serve as candidate for molecular characterization of GBM. The authors have used a selective sig1R ligand (S)-(−)-[18F]fluspidine to test this hypothesis with PET noninvasive molecular imaging.
In conclusion, the data obtained in the U87-MG mouse model of GBM along with the detection of sig1R in human GBM tissue for the first time by a PET radioligand, indicate not only the relevance of this target but also the suitability of (S)-(−)-[18F]fluspidine for sig1R-targeted cancer research and drug development.
Results from nanoScan PET/MRI
- Dynamic PET imaging showed that the uptake of (S)-(−)-[18F]fluspidine has higher retention in the tumor region compared to the CL at 60 min p.i., with SUVs of 0.38 and 0.28, respectively (Figure 4.)

Figure 4. PET/MR imaging of sig1R in mice with orthotopic xenograft of human GBM cells (U87-MG). Average time-activity curves after i.v. administration of (S)-(−)-[18F]fluspidine of the tumor (red dots) and the contralateral (black squares) regions of interest (n = 3). Statistical test: Student t-test, * p < 0.05.
- The early dynamic PET images between 2 and 9 min after injection show a heterogeneous uptake of (S)-(−)-[18F]fluspidine into the tumor (Figure 5D, upper panel), which may be caused by reduced blood supply to the tumor center. The PET image at later time points (45 to -60 min p.i.; Figure 5D, lower panel) pictures a more homogenous uptake of the tracer, along with a low slope, reflecting an accumulation.

Figure 5. (D) Representatives coronal PET/MR images of U87-MG tumor-bearing mouse after i.v. administration of (S)-(−)-[18F]fluspidine. The upper panel exhibits the distribution of (S)-(−)-[18F]fluspidine at early times p.i. (averaged time frames from 2 to 9 min), and the lower panel exhibits the distribution of (S)-(−)-[18F]fluspidine at later times (averaged time frames from 45 to 60 min). The regions-of-interest (ROIs) were delineated on the T2-weighted MR images and then applied on the PET data to generate the regional TACs.
Simultaneous Visualization of 161Tb- and 177Lu-Labeled Somatostatin Analogues Using Dual-Isotope SPECT Imaging
Francesca Borgna1, Patrick Barritt1, Pascal V. Grundler1, Zeynep Talip1, Susan Cohrs1, Jan Rijn Zeevaart2, Ulli Köster3, Roger Schibli1,4, Nicholas P. van der Meulen1,5 and Cristina Müller1,4
1 Center for Radiopharmaceutical Sciences, Paul Scherrer Institute, 5232 Villigen-PSI, Switzerland
2 Radiochemistry, South African Nuclear Energy Corporation (Necsa), Brits 0240, South Africa
3 Institut Laue-Langevin, 38042 Grenoble, France
4 Department of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland
5 Laboratory of Radiochemistry, Paul Scherrer Institute, 5232 Villigen-PSI, Switzerland
https://doi.org/10.3390/pharmaceutics13040536
Summary
177Lu (half-life 6.7d) is currently the most often applied radiometal for therapeutic purposes, as it has a particulate emission (β− or Auger electron) for effecting therapy and emits several accompanying γ-photons of 208 keV (11%) and 113 keV (6.4%), which are used for diagnostic evaluation and dosimetry.
161Tb is a more recently introduced radiolanthanide for therapeutic applications. 161Tb decays with a half-life of 6.89 days to stable 161Dy, while emitting β¯-particles (Eβ͞av = 154 keV) suitable for therapeutic purposes and γ-radiation (Eγ = 49 keV, I = 17.0%; Eγ = 75 keV, I = 10.2%) useful for SPECT imaging. 161Tb also emits a substantial number of low-energy conversion and Auger electrons, which makes this radionuclide exceptionally interesting for the treatment of disseminated cancers with multiple metastases ranging from a single cell (diameter: ~10μm) to micro cell clusters (diameter: < 1mm). Monte Carlo simulations assessed the dose delivered to 10μm spheres revealed a 3.5-fold increased value when using 161Tb as compared to 177Lu. In larger tumors (diameter > 10mm), the emitted electron energy from 161Tb and 177Lu respectively is almost entirely absorbed, resulting in a 1.3-fold higher absorbed electron energy fraction per decay for 161Tb compared to 177Lu, making 161Tb more potent than 177Lu.
The aim of the present study was to use dual-isotope SPECT imaging in order to demonstrate that 161Tb and 177Lu are interchangeable without compromising the pharmacokinetic profile of the radiopharmaceutical.
After in vitro characterization, 161Tb- and 177Lu-labeled somatostatin (SST) analogues DOTATOC (agonist) and DOTA-LM3 (antagonist) were injected to AR42J tumor-bearing nude mice. In vivo disptribution profiles were investigatd by dual-isotope SPECT/CT imaging. Results revealed identical pharmacokinetic profiles of the two peptides, irrespective of whether it was labeled with 161Tb or 177Lu. Moreover, the visualization of the sub-organ distribution confirmed similar behavior of 161Tb- and 177Lu-labeled SST analogues. These and previous findings suggest that any future (pre)clinical studies with 161Tb can be based on preclinical data obtained with its 177Lu-labeled counterpart. This will allow the focusing of future investigations directly on the therapeutic efficacy of 161Tb, which is likely to be superior to the effect obtained with 177Lu.
Results from nanoSPECT/CT
Five-week-old female CD1 nude mice were subcutaneously inoculated with AR42J tumor cells (5x106 cells in 100µl PBS). The scans were performed 10–14 days after tumor cell inoculation when the tumor size reached a volume of ~250mm3.
Mice were i.v. injected with a mixture of 161Tb-DOTATOC (~15MBq) and 177Lu-DOTATOC (~15MBq) or a mixture of 161Tb-DOTA-LM3 and 177Lu-DOTA-LM3 (~30MBq) at a 161Tb/177Lu activity ratio of 1:1. For specificity test, blocking studies were performed under the same experimental conditions; however, in this case, an excess of unlabeled DOTATOC or DOTA-LM3 was added to the injection solution. SPECT/CT scans were acquired 2h, 4h, and 24h after injection of the radiopeptides using the dual-isotope SPECT acquisition protocol with a frame time of 60s resulting in a scan time of 45min.
For the SPECT/CT scan, simultaneous acquisition of counts stemming from 161Tb and 177Lu, respectively, was performed by the selection of distinct energy windows for the two radionuclides. The two energy windows chosen for 161Tb were set at 47.7keV±10%, which enabled the detection of X-rays and γ-rays (46.0keV, 48.9keV and 52.0keV), and at 74.6keV±10%, enabling the detection of the γ-rays at 74.6keV. For 177Lu, the windows were set at 112.9keV±10% and 208.4±10% to detect the γ-rays. Prior to animal studies, phantom scans were carried out in order to verify of the dual-isotope SPECT imaging protocol using Eppendorf vials filled with 161Tb or 177Lu or both: analysis revealed no interference between 161Tb and 177Lu in the acquired scans; each radionuclide was visualized independently of the other with high accuracy.
Analysis of the SPECT/CT images revealed:
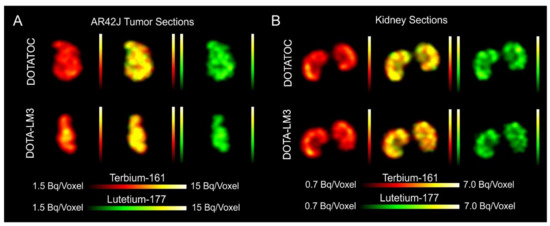
- Equal in vivo distribution of simultaneously injected 161Tb-DOTATOC and 177Lu-DOTATOC. The same observation was made for 161Tb-DOTA-LM3 and 177Lu-DOTA-LM3. Images reconstructed using the energies of either radiolanthanide (red-to-yellow scale and green-to-yellow scale for 161Tb and 177Lu, respectively), provided the distribution for each radiopeptide separately in the same mouse.
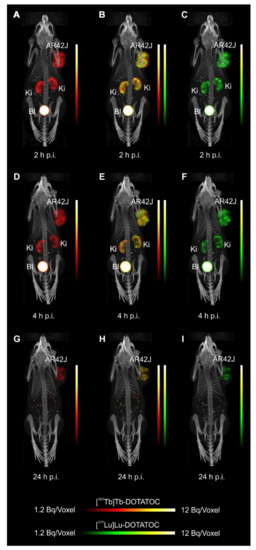
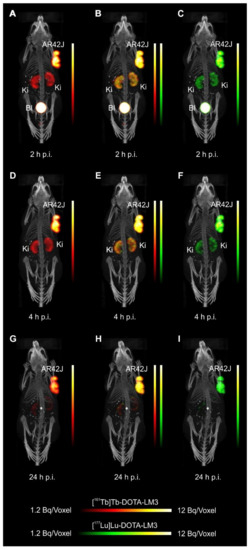
- Experiments performed by co-injection of excess unlabeled peptide resulted in SSTR blockade and, hence, accumulation of the radiopeptides in AR42J tumors was not observed. These additional studies proved that the uptake of the SST analogues in AR42J tumor xenografts was SSTR-specific.
- Quantification of the accumulated activity in AR42J tumors and kidneys confirmed equal distribution of the 161Tb- and 177Lu-labeled counterparts. This was the ultimate proof that the chosen radiolanthanide did not have an impact on the tissue distribution profile of the radiopeptides.
- The SPECT/CT images showed activity accumulation in the AR42J xenografts, which was higher for the antagonist than for the agonist. In agreement with quantitative data from biodistribution studies, the activity was efficiently cleared through the kidneys over time and almost entirely excreted after 24h. Due to the favorable uptake of radiolabeled DOTA-LM3 in the tumor tissue, the tumor-to-kidney ratio was higher as compared to the ratio obtained after injection of radiolabeled DOTATOC.
- Dual-isotope SPECT image sections enabled, for the first time, visualization of the 161Tb- and 177Lu-labeled peptide distribution at a sub-organ level in the same animal. Most important to note is that the pattern of activity distribution in tumors and kidneys was the same, irrespective of whether 161Tb or 177Lu was used. The uptake in the tumor was quite homogenous, which can be ascribed to the well-vascularized AR42J xenograft. Accumulation of activity in the kidneys was more prominent in the cortex where the megalin-mediated reabsorption of radiopeptides occurs, and where various SSTR subtypes are known to be expressed
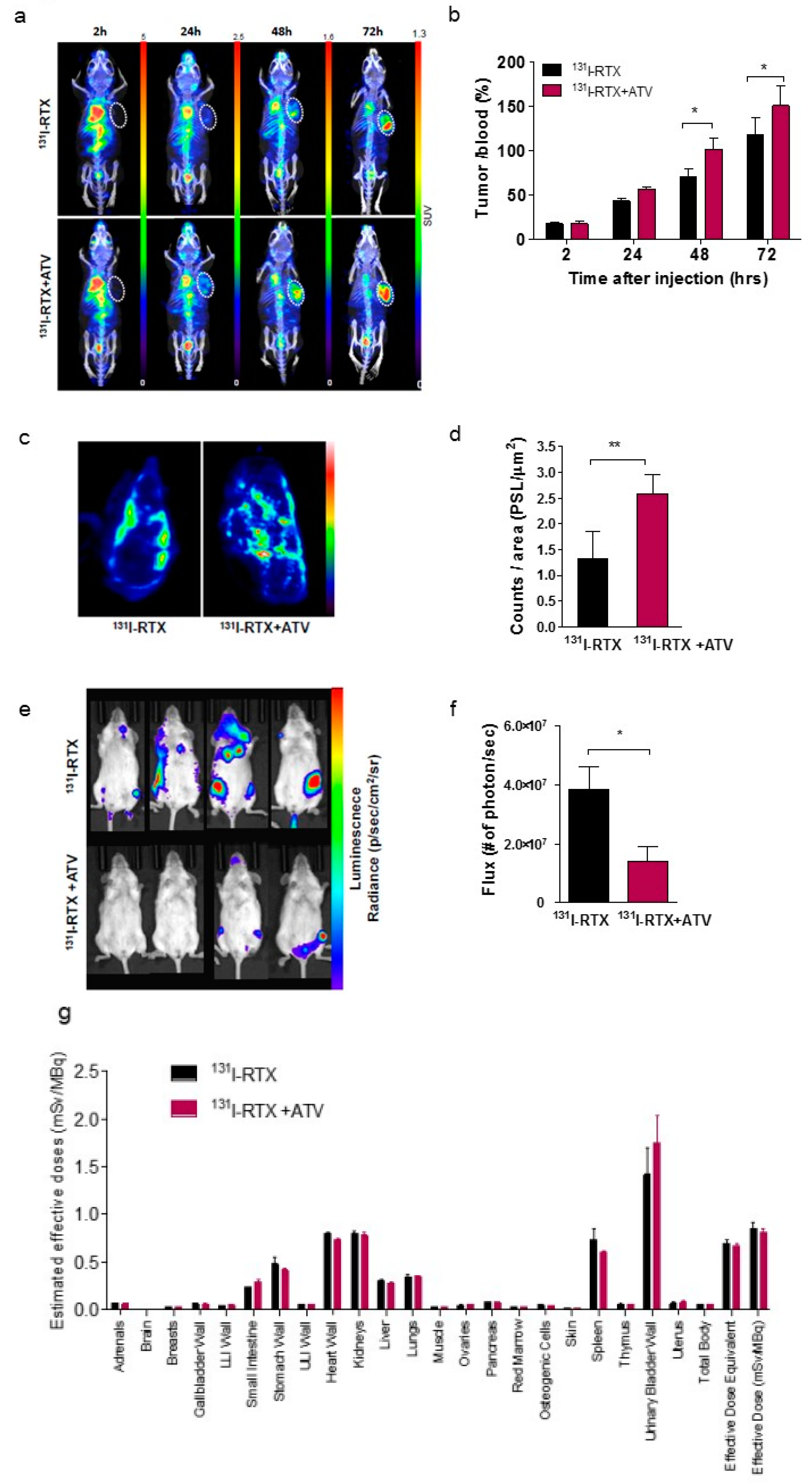
Inhibition of HIF-1α by Atorvastatin During 131I-RTX Therapy in Burkitt’s Lymphoma Model
Eun-Ho Kim1,2, Hae Young Ko3,4, A Ram Yu5, Hyeongi Kim3, Javeria Zaheer3,6, Hyun Ji Kang3,6, Young-Cheol Lim3, Kyung Deuk Cho3, Hyun-Yoo Joo1, Min Kyoung Kang5, Jae Jun Lee5, Seung-Sook Lee7, Hye Jin Kang8, Sang Moo Lim3,9, Jin Su Kim3,6
A non-human primate model for stable chronic Parkinson’s disease induced by MPTP administration based on individual behavioral quantification
https://doi.org/10.1016/j.jneumeth.2018.10.037
Summary
Parkinson’s disease (PD) is a neurodegenerative disease that affects about 3% of the population over 65 years of age. PD is characterized by a selective loss of dopaminergic neurons accompanied by movement disorders, including rigidity, bradykinesia, akinesia, tremors, and postural instability.
The authors aimed to develop a MPTP-induced chronic NHP model with stable symptoms by adjusting MPTP treatment based on individual behavioral quantification using a video-based analysis system. To validate this strategy, they assessed a parkinsonian behavior score, an immunohistochemistry Western blot, and PET imaging of dopamine transporter (DAT) with [18F] N-(3-fluoropropyl)-2ß-carboxymethoxy-3β-(4-iodophenyl) nortropane (18F-FP-CIT).
The authors stated that the novel strategy of MPTP administration based on global activity evaluations using a video-based analysis system provides an important conceptual advance of this method for the development of chronic NHP PD models.
Results from nanoScan PET/CT
Mediso’s nanoScan PET/CT with 16cm bore size, 12 cm transaxial and 10 cm axial Field of View (FOV) were suitable to execute PET/CT scans in cynomolgus monkeys (Macaca fascicularis). Furthermore high spatial and temporal resolution of PET allowed to determine the dopamine transporter biding potential (BP) in different striatal sub-regions.
Following a CT scan for attenuation correction, 185 MBq 18 F-FP-CIT in 1.5 ml saline was injected intravenously via the saphenous vein. To monitor presynaptic dopamine transporter activity in vivo, serial 18F-FP-CIT PET imaging was performed after MPTP administration.
- The biding potential analysis revealed that the 18F-FP-CIT BP were significantly reduced in all sub-regions of the striatum at 8, 16, 24, 32,40 and 48 weeks following the first MPTP administration (Fig. 4A and 4B).
- Monkeys with fewer striatal DAT tended to have lower global activity (GA), indicating that a decrease in GA reflects damage in dopaminergic neurons.
- The severity of Parkinsonian symptoms and the loss rates of DAT in each striatal sub-region did not correlate with the total dose of MPTP, indicating that a fixed MPTP dose is not a good strategy for development of chronic NHP PD models

Fig. 4. (A) Representative 18F-FP-CIT PET images fused with individual MRI. (B) Histogram representing 18F-FP-CIT binding potential (BP) in the MPTP-injection group. (#P < 0.05, *P<0.01 vs. baseline). (C) Histogram representing time activity curve. The radiotracer uptake in each region of interest was estimated as the standardized uptake value (SUV), which was calculated as decay-corrected activity per milliliter of tissue volume per injected radiotracer activity per body mass ([kBq/mL]/[kBq/g]). Ant, anterior; binding potential, BP; Ext, external; pallidum, globus pallidus; Int, internal; Post, posterior.
Iodine‑124 PET quantification of organ‑specific delivery and expression of NIS‑encoding RNA
Matthias Miederer1, Stefanie Pektor1, Isabelle Miederer1, Nicole Bausbacher1, Isabell Sofia Keil2,
Hossam Hefesha3, Heinrich Haas3, Ugur Sahin2,3 and Mustafa Diken2,3
1 Department of Nuclear Medicine, University Medical Center of Johannes Gutenberg University, Mainz, Germany
2 TRON - Translational Oncology at the University Medical Center, Johannes Gutenberg University Mainz GmbH, Mainz, Germany
3 Biopharmaceutical New Technologies (BioNTech) SE, Mainz, Germany
https://doi.org/10.1186/s13550-021-00753-2
Summary
There has been increased interest in the development of mRNA-based vaccines for protection against various infectious diseases and also for cancer immunotherapies since lipid-based nanoparticles opened the possibility to deliver RNA to specific sites within the body, overcoming the limitation of rapid degradation in the bloodstream. In the present study, RNA-lipoplex nanoparticles were assembled by complexing sodium-iodide-symporter (NIS) coding mRNA with liposomes at different charge ratios. Two kinds of RNA-lipoplex systems were used: one system with net anionic charge mediating translation primarily within the spleen, and the other with net positive charge yielding translation primarily within the lungs. After in vitro analysis of the expression kinetics, mice were iv. injected with the mRNA-lipoplexes then 6h later with 124Iodine. Functional NIS protein translation was investigated by PET/MRI imaging. Results revealed rapid increase of 124Iodine uptake in the spleen or lung compared to control-RNA-lipoplexes (containing non-coding RNA) with minimal background in other organs except from thyroid, stomach and salivary gland (where NIS is physiologically expressed). The strong organ selectivity and high target-to-background acquisition of NIS-RNA lipoplexes indicate the feasibility of small animal PET/MRI to quantify organ-specific delivery of RNA.
Results from nanoScan PET/MRI
Female BALB/c mice were intravenously injected with RNA-lipoplexes containing 20μg NIS RNA. Six hours later 6.64±0.66MBq 124Iodine was injected intravenously. Three hours after 124Iodine injection, mice were anesthetized and static imaging was performed over 20min by nanoScan PET/MRI. Additionally, one animal per group was imaged dynamically for one hour.
- PET/MRI of anionic NIS-RNA lipoplexes showed a visually detectable increase of 124Iodine uptake in the spleen compared to control-RNA lipoplexes. Due to the high physiological NIS expression in the adjacent gastric wall, this increase was only visually clear with anatomical correlation by MRI. On PET imaging, spleen uptake appeared as an irregularity of the gastric wall which is not detected in control animals
- Lung uptake of NIS-RNA transported by cationic RNA-lipoplexes was depicted more clearly due to larger organ size and no adjacent physiological NIS uptake
- The quantified radioactivity from imaging matched well with the extent of uptake as measured in organs ex vivo, showing enhanced uptake of NIS-RNA and expression of functional NIS-protein in lung or spleen compared to the control RNA
- The uptake in lung was rapid and remained high over the first hour of dynamic acquisition

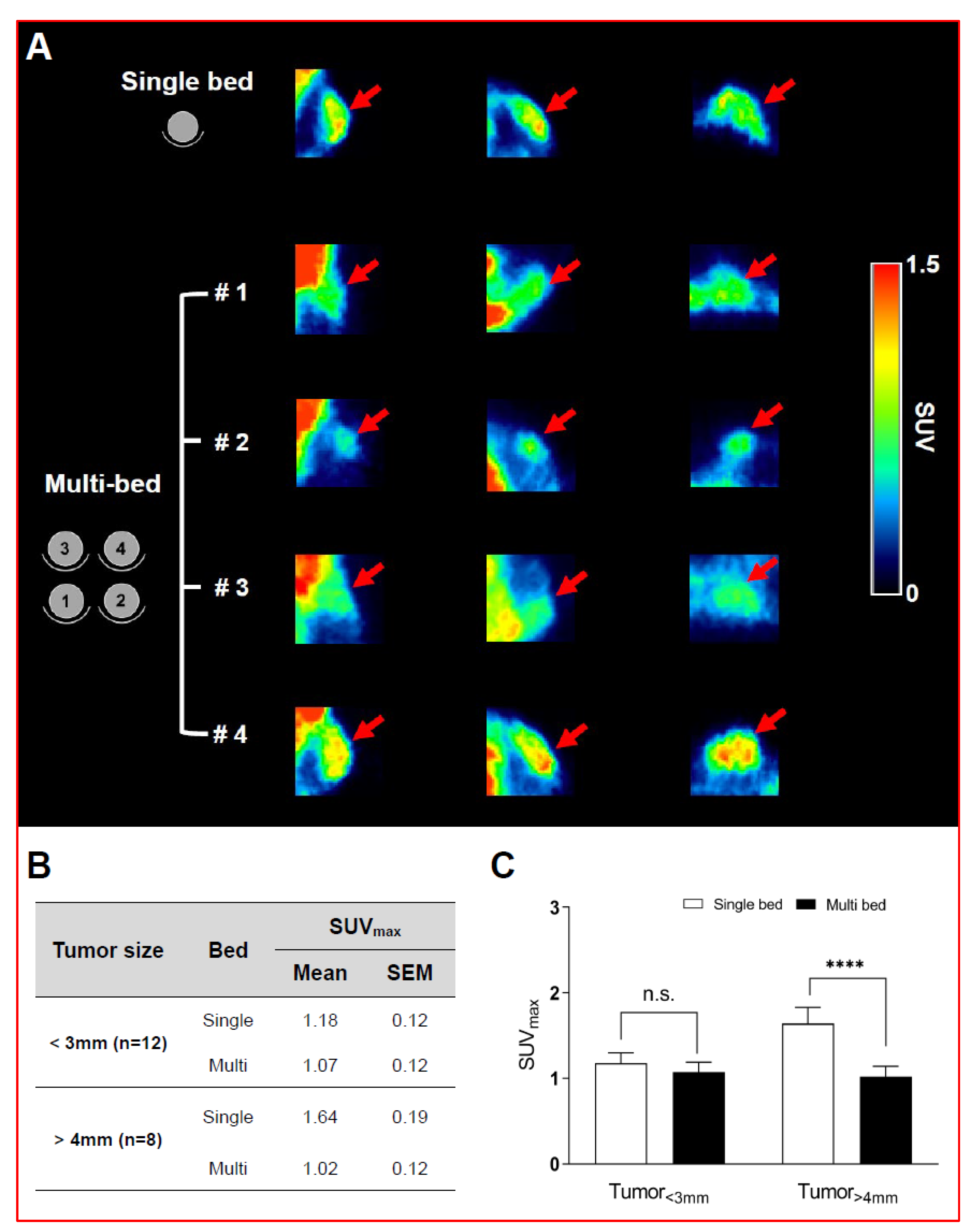
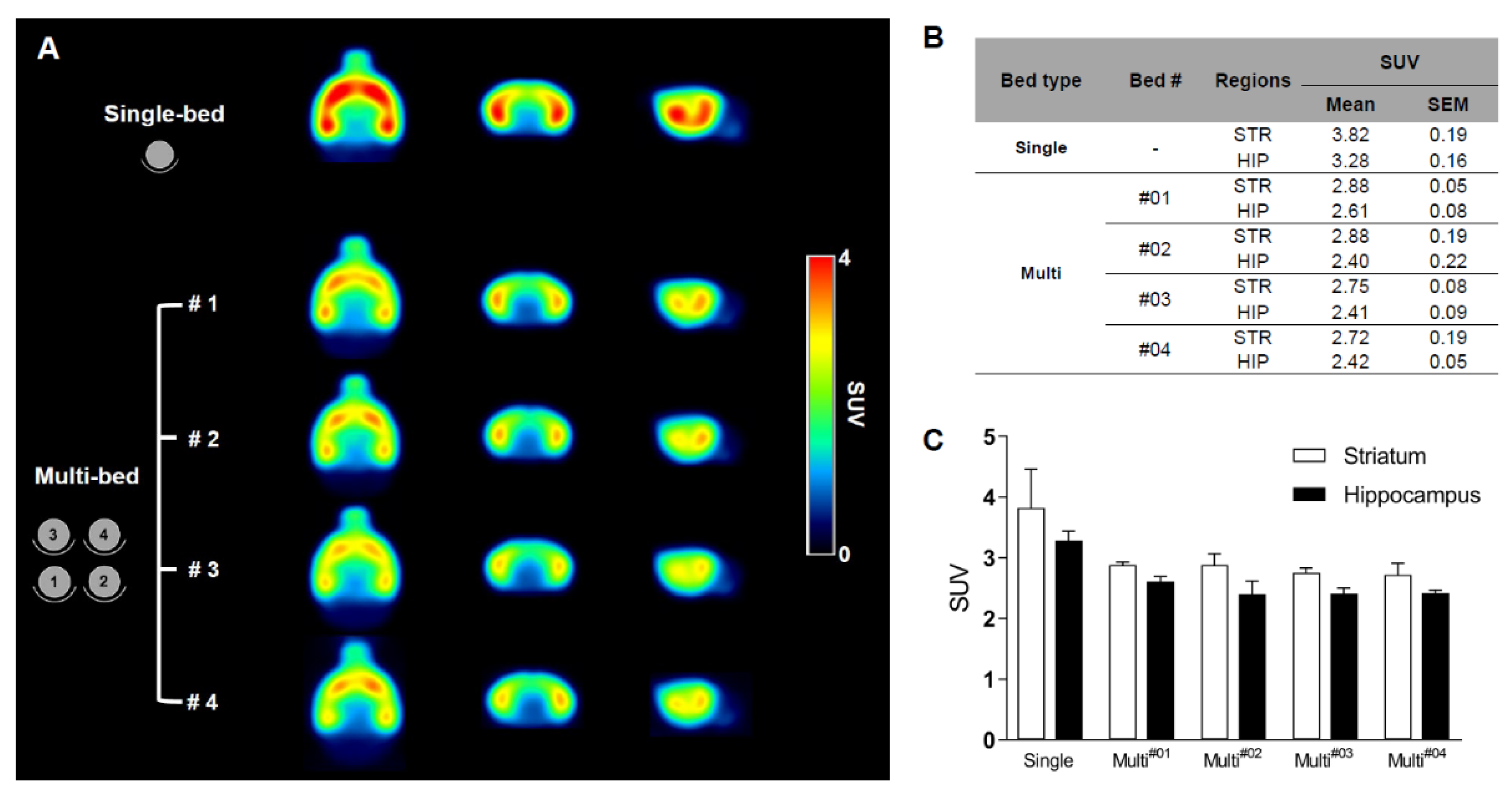
Validation of Image Qualities of a Novel Four-Mice Bed PET System as an Oncological and Neurological Analysis Tool
Kyung Jun Kang1, Se Jong Oh1, Kyung Rok Nam1, Heesu Ahn1,2, Ji-Ae Park1,2, Kyo Chul Lee1, Jae Yong Choi1,2
1Division of Applied RI, Korea Institute of Radiological and Medical Sciences, Seoul 01812, Korea
2Radiological and Medico-Oncological Sciences, University of science and technology (UST), Seoul 01812, Korea
https://doi.org/10.3390/jimaging7030043
For this purpose, researchers use micro-PET (µPET), which is a small-animal dedicated system. Most µPET vendors provide a single bed, thereby allowing imaging of only a single animal at a time. Large-scale research involving many objects thus requires tremendous time and use of radioactivity.
Recently, Mediso developed a multi-bed system dedicated to the nanoScan scanners with the contribution of Tim Witney and his team at UCL and KCL, where the initial validity for research has been investigated. Greenwood et al. (https://doi.org/10.2967/jnumed.119.228692) tested the four-bed mouse system using 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) in phantom and normal mice and reported a quantitative accuracy similar to that of a single-bed. To date, however, few studies have focused on the validity of oncological and neurological PET imaging of the four-mice bed system.
This study aimed to evaluate the image qualities of oncological and neurological PET imaging using a novel four-mice bed system.
Adjuvanting a subunit SARS-CoV-2 nanoparticle vaccine to induce protective immunity in non-human primates
 https://www.biorxiv.org/content/10.1101/2021.02.10.430696v1
https://www.biorxiv.org/content/10.1101/2021.02.10.430696v1
Summary
Despite of the success of messenger RNA vaccines against SARS-CoV-2, a wider portfolio of different vaccine candidates would be needed to stop COVID-19 pandemic. In particular, vaccinating infants and the elderly could benefit from the use of subunit vaccine platforms with a demonstrable history of safety and efficacy in such populations.
Subunit vaccines include only a fragment of a pathogen which can still induce the immune system. Although, the production of these types of vaccines are safer than producing attenuated pathogen, they often require adjuvants to enhance the immune response for long term protection.
In this extensive research collaboration, the authors demonstrate the capacity of a subunit vaccine under clinical development, containing the SARS-CoV-2 Spike protein receptor binding domain displayed on a two component protein nanoparticle (RBD-NP). They assessed the immunogenicity and protective efficacy of RBD-NP vaccination using five different clinically relevant adjuvants in non-human primates.
Among the tested adjuvants, AS03, an alpha-tocopherol-containing squalene-based oil-in-water emulsion, and CpG 1018 (with Alum), a Toll-like receptor 9 agonist formulated in Alum, were the most promising adjuvants.
The team concluded that the neutralizing antibody response by the RBD-NP/AS03 vaccination was durable. The study results can also help in the development of subunit vaccines to combat the ongoing pandemic.
Results from MultiScan LFER PET/CT
The researchers in University of Pittsburgh School of Medicine evaluated inflammation in the lung tissues with no adjuvant, AS03 and CpG-Alum on pre and post challenge days using MultiScan LFER PET/CT
- Vaccinated animals showed FDG uptake, to a much lesser extent than the control animals (Fig. 3e and f)

Fig 3 e, Inflammation in the lungs of two animals from each group indicated in the legend, pre-challenge (day 0) and post-challenge (day 4 or 5 after infection), measured using PET-CT scans. f, Representative PET-CT images of lungs from one animal in each group. PET signal is scaled 0 to 15 SUV.
SPECT imaging evaluation of 111indium-chelated cetuximab for diagnosing EGFR-positive tumor in an HCT-15-induced colorectal xenograft
Bin-Bin Shiha, Yi-Fang Changb, Chun-Chia Chengb, Hao-Jhih Yangc, Kang-Wei Changc, Ai-Sheng Hoa, Hua-Ching Lind, Chun Yeha, Chun-Chao Change,f
a Division of Gastroenterology, Cheng Hsin General Hospital, Taipei, Taiwan, ROC
b Hematology and Oncology, Mackay Memorial Hospital, Taipei, Taiwan, ROC
c Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan, ROC
d Division of Proctology, Cheng Hsin General Hospital, Taipei, Taiwan, ROC
e Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei, Taiwan, ROC
f Division of Gastroenterology and Hepatology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, ROC
http://dx.doi.org/10.1016/j.jcma.2017.02.010
Summary
Colorectal cancer (CRC) occurs with high incidence worldwide, but is usually diagnosed in late stage with metastasis by the conventional methods. Epidermal growth factor receptor (EGFR) is overexpressed in 97% of CRC cells, serving a promising diagnostic candidate. In the present study, Cetuximab, an anti-EGFR monoclonal antibody was conjugated with an isotope chelator, diethylene triamine penta acetic acid (DTPA), labeled with 111indium (111In) and injected to tumor bearing mice. Biological distrubution was investigated by SPECT/CT imaging.
Results revealed that 111In-Cetuximab accumulated in the both small (50mm3) and large (250mm3) tumors, whereas the ratio of tumor to muscle in the large tumor was 7.5-fold. The biodistribution data indicated that the 111In-cetuximab bound to tumor specifically that was higher than that in other organs. Consequently, 111In-cetuximab is suggested to be suitable for early diagnosis and prognostic monitor of EGFR-positive CRC in further clinical practice.
Results from nanoSPECT/CT
- The tumor of the 111In-Cetuximab group was apparently observed both in 24h and 48h and higher than that in the 111In group.
- 111In-Cetuximab majorly accumulated in liver and tumor, otherwise, 111In accumulated only in the kidney.
- The tumor to muscle ratio of 111In-Cetuximab was measured 7.5-fold, which was higher than that of 111In group measured as 3.1-fold, indicating that 111In-cetuximab specifically bound to EGFR-positive tumors as a reliable diagnosing agent.
- The result also indicated that 111In labeled with Cetuximab through chelator DTPA was easily excreted out the mice better than free 111In, suggesting that this labeling method may not lead to accumulation of 111In metal in mice.

In vivo imaging with 18F-FDG- and 18F-Florbetaben-PET/MRI detects pathological changes in the brain of the commonly used 5XFAD mouse model of Alzheimer's Disease
Copper‑67 radioimmunotheranostics for simultaneous immunotherapy and immuno‑SPECT
Guiyang Hao1, Tara Mastren1, William Silvers1, Gedaa Hassan1, Orhan K. Öz1 &
Xiankai Sun1,2
1 Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.
2 Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.
![]() ttps://www.nature.com/articles/s41598-021-82812-1
ttps://www.nature.com/articles/s41598-021-82812-1
Introduction
Copper-67 is useful from both therapeutic and diagnostic standpoints due to its medium energy beta particle, gamma emissions, and 2.6-day half-life. Moreover, since copper radioisotopes are chemically identical, the same radiopharmaceutical can be used for 64Cu PET imaging to pre-screen of patients and 67Cu based radionuclide therapy. For these reasons, the usage of 67Cu in radiotherapy has long been arisen. However until now, its widespread use has been limited by unreliable supplies, cost, and difficulty in obtaining therapeutic quantities. The recent breakthrough of copper-67 production provides an opportunity to reassess its use in radiotherapy.
Summary
In this work, Xiankai Sun and his co-workers have evaluated and demonstrated the practical use of 67Cu in radioimmunotherapy. To demonstrate the concept, human epidermal growth factor receptor 2 (HER2) antibody, pertuzumab, was labeled with 67Cu isotope. The radiolabeling efficiency was two-order of magnitude higher compared to literature reports. Mice bearing HER2+ xenografts showed 67Cu-dose dependent tumor-growth inhibition from 67Cu-labeled-Pertuzumab co-administered with trastuzumab. Moreover, authors visualized and measured [67Cu]Cu-NOTA-Pertuzumab uptake quantitatively by SPECT imaging.
Results from nanoSPECT/CT
Thanks to technological advances of Mediso's SPECT/CT, researchers were able to perform quantitative data analysis.
- All the tumors were clearly visualized with [67Cu]Cu-NOTA-Pertuzumab on both day 2 and day 5 post injection (Fig. 4A).
- Quantitative tumor uptake from the SPECT imaging data are presented as absolute radioactivity concentration (μCi/mL) (Fig. 4B)

Figure 4. SPECT/CT imaging results. (A) Representative maximum intensity projection (MIP) SPECT/CT images of HCC1954 HER2+ tumor-bearing mice injected with [67Cu]Cu-NOTA-Pertuzumab (Group 2, 3, 4, and 5) at day 2 and 5 post the start of treatment (yellow arrows indicate the tumors); (B) Actual radioactivity concentration in tumors (MBq/mL) on Day 2 and 5 (without decay correction).
Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1
Anzhelika Vorobyeva1,2,† , Elena Konovalova3,†, Tianqi Xu1, Alexey Schulga2,3, Mohamed Altai1, Javad Garousi1, Sara S. Rinne4, Anna Orlova2,4,5, Vladimir Tolmachev1,2 and Sergey Deyev2,3,6,7
1 Department of Immunology, Genetics and Pathology, Uppsala University, 751 85 Uppsala, Sweden
2 Research Centrum for Oncotheranostics, Research School of Chemistry and Applied Biomedical Sciences, Tomsk Polytechnic University, Tomsk, Russia
3 Molecular Immunology Laboratory, Shemyakin & Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia
4 Department of Medicinal Chemistry, Uppsala University, Uppsala, Sweden
5 Science for Life Laboratory, Uppsala University, Uppsala, Sweden
6 Bio-Nanophotonic Lab, Institute of Engineering Physics for Biomedicine (PhysBio), National Research Nuclear University ‘MEPhI’, Moscow, Russia
7 Center of Biomedical Engineering, Sechenov University, Moscow, Russia
† A.V. and E.K. contributed equally
https://doi.org/10.3390/ijms21093310
Summary
Up to 85% of ovarian cancer patients are diagnosed only at advanced stages, when cancer has already spread through the body. The present study aimed to find a more efficient way for diagnosis and treatment using mouse xenograft models.
As epithelial cell adhesion molecule (EpCAM) is overexpressed in 55%–75% of ovarian carcinomas (OC), it might be a promising target. Designed ankyrin repeats protein (DARPin) Ec1 binds to EpCAM with subnanomolar affinity. In the present study, DARPin Ec1 was labeled with 125I using N-succinimidyl-para-iodobenzoate (PIB) and injected to mice bearing SKOV-3 or OVCAR-3 xenografts. In vitro experiments showed highly specific binding to ovarian carcinoma cells, moreover, slow internalization, which is essential for in vivo imaging a few hours after injection. In vivo biodistribution analyses of SPECT/CT images suggest that EpCAM on ovarian cancer xenografts is sufficiently accessible to permit DARPin-mediated delivery of cytotoxic payload.
Results from nanoScan SPECT/CT
For establishment of xenografts, 107 of SKOV-3 and OVCAR-3 cells or 5x106 Ramos cells (EpCAM-negative lymphoma xenografts served as specificity control) in 100µl of media were subcutaneously injected in the right hind leg of female Balb/c nu/nu mice. The experiments in mice bearing SKOV-3 and Ramos xenografts were performed 2–3 weeks after implantation. The experiments in mice bearing OVCAR-3 xenografts were performed 7 weeks after implantation.
Mice were injected with 125I-PIB-Ec1 (20µg, 1.2MBq for SKOV-3, and 6µg, 2.8MBq for OVCAR-3), SPECT/CT images were acquired 6h pi time later for 20min. with nanoScan SPECT/CT.
- In vitro studies revealed specific binding to SKOV-3 and OVCAR-3 cells; rapid binding and slow dissociation and internalization
- SPECT/CT imaging demonstrated that radiolabeled 125I-PIB-Ec1 provided clear visualization of both EpCAM-expressing xenografts. In vivo biodistribution is characterized by high tumor-to-organ ratio, the only organ with noticeable activity were kidneys.

Iodine-124 PET quantification of organ-specific delivery and expression of NIS-encoding RNA
Matthias Miederer1, Stefanie Pektor1, Isabelle Miederer1, Nicole Bausbacher1, Isabell Sofa Keil2, Hossam Hefesha3, Heinrich Haas3, Ugur Sahin2,3, Mustafa Diken2,3
1Department of Nuclear Medicine, University Medical Center of Johannes Gutenberg University, Mainz, Germany
2TRON - Translational Oncology at the University Medical Center, Johannes Gutenberg University Mainz gGmbH, Mainz, Germany
3Biopharmaceutical New Technologies (BioNTech) SE, Mainz, Germany
https://doi.org/10.1186/s13550-021-00753-2
Summary
RNA-based vaccination strategies tailoring immune response to specific reactions have become an important pillar for a broad range of applications. Recently, the use of lipid-based nanoparticles opened the possibility to deliver RNA to specific sites within the body, overcoming the limitation of rapid degradation in the bloodstream.
Nanoparticles show promising potency as delivery vehicles for a variety of molecules, leading to fundamentally new applications and therapeutic strategies. Due to their complex chemical composition and relevant interaction with plasma proteins, the pharmacokinetic properties and delivery properties of nanoparticles are variable and remain challenging to adapt for optimal conditions. Accomplishing precise RNA delivery to target tissue using nanoparticles would serve as a versatile platform that enables easy and transient expression of any protein in principal. RNA is currently already in use to selectively activate the immune system against specific target proteins for cancer therapy.
In the article, the authors have investigated whether small animal PET/MRI can be employed to image the biodistribution of RNA-encoded protein. For this purpose, a reporter RNA coding for the sodium-iodide-symporter (NIS) was assembled with liposomes at different charge ratios, and functional NIS protein translation was imaged and quantified in vivo and ex vivo by Iodine-124 PET upon intravenous administration in mice.
Results from the nanoScan PET/MRI
For the small animal imaging, the authors have used a nanoScan PET/MRI 1T, which provided a perfect option to follow the uptake of Iodine-124 not just in the thyroids, but also in the NIS-expressing tissues. Moreover, it could be accompanied by the help of MRI, to identify internal organs, like spleen.
Groups of n = 3 animals were intravenously injected with RNA-lipoplexes containing 20 µg NIS RNA. Six hours later 6.64 ± 0.66 MBq Iodine-124 was injected intravenously. Three hours after Iodine-124 injection, mice were anesthetized with 2% isoflurane and static imaging was performed over 20 min. For anatomic imaging MRI measurements (Material Map for coregistration of the PET scan; 3D Gradient Echo External Averaging (GRE-EXT), Multi Field of View (FOV); slice thickness: 0.6 mm; TE: 2 ms; TR: 15 ms; flip angle: 25 deg) were performed afterward. Additionally, one animal per group was imaged dynamically for one hour. PET data were reconstructed with Teratomo 3D (4 iterations, 6 subsets, voxel size 0.4 mm), co-registered to the MR and corrected for decay.
Figure 2. shows the PET/MRI of Iodine-124 distribution in vivo. (A) Coronal slices of PET/MRI fusion and volumes of interests (red) for spleen and lung are shown in representative animals. From left to right: targeting of spleen with non-coding RNA, targeting of spleen with NIS RNA, targeting of lung with non-coding RNA, targeting of lung with NIS-RNA. (B) Calculated organ uptake from the volumes of interests. Data are shown as mean + SD of n = 3 mice. (C) Representative in vivo bioluminescence images of Luc-RNA lipoplexes after targeting the spleen and lung. (D) Maximum intensity projections of PET images after application of lung-targeting NIS-RNA lipoplexes (right) in comparison with non-coding control (left). (E) Time activity curve of Iodine-124 uptake in the lung over 60 min immediately after Iodine-124 injection (6 h after administration of NIS-RNA lipoplexes targeting the lung).
- In this study, two RNA-lipoplex systems for systemic NIS-RNA delivery were compared by small animal PET/MRI of Iodine-124 uptake. One system with net anionic charge is known to mediate translation primarily within the spleen, and the other with net positive charge is known to yield translation primarily within the lungs.
- Tha authors have shown highly specific targeting, delivery and expression of RNA to spleen and lung by anionic and cationic RNA-lipoplex nanoparticles, respectively, through the use of the NIS reporter gene system and Iodine-124 uptake as imaged by PET/MRI. Combining NIS reporter gene imaging with in vivo small animal PET/MRI thus represents a powerful tool to monitor the distribution and extent of expression of RNA targeted specifically to any tissue over time.

PD-1 blockade exacerbates Mycobacterium tuberculosis infection in rhesus macaques
Keith D Kauffman, Shunsuke Sakai, Nickiana E Lora, Sivaranjani Namasivayam, Paul J Baker, Olena Kamenyeva, Taylor W Foreman, Christine E Nelson, Deivide Oliveira-de-Souza, Caian L. Vinhaes, Ziv Yaniv, Cecilia S Lindestam Arleham, Alessandro Sette, Gordon J Freeman, Rashida Moore, the NIAID/DIR Tuberculosis Imaging Program, Alan Sher, Katrin D Mayer-Barber, Bruno B Andrade, Juraj Kabat, Laura E Via, Daniel L Barber
doi: 10.1126/sciimmunol.abf3861, ![]() BioRxiv
BioRxiv
Summary
PD-1 (programmed death-1) is a coinhibitory receptor primarily expressed on activated CD4 and CD8 T cells that has been shown to limit the function of pathogen-specific T cells during chronic infection. The reactive expression of PD-L1 (PD-1 receptor ligand) on cancer cells turns off the T cells that are trying to attack the tumor. Therefore, blockade of PD-1 receptor or its ligands with monoclonal antibodies (mAbs) has become an attractive target in cancer therapy. Recognition of this pathway has led to suggestions that anti–PD-1 therapy might also boost T cell immunity in chronic infections including tuberculosis.
In this recent Science Immunology publication, Kauffman et al. examined the role of PD-1 during Mycobacterium tuberculosis (Mtb) infection of rhesus macaques. It was shown that the PD-1 blockade increased the number and functionality of Mtb-specific CD8 T cells, but not CD4 cells and was associated with increases in proinflammatory cytokines. However, animals treated with anti–PD-1 monoclonal antibody developed worse disease and higher granuloma bacterial loads compared with isotype control–treated monkeys.
These data indicate that negative regulation of immune responses is a critical aspect of host resistance to Mtb infection. Also, these findings suggest that the anti-PD-1 cancer therapy needs to be used cautiously in patients with a history of Mtb exposure.
Results from MultiScan LFER
Mediso MultiScan™ Large Field of view Extreme Resolution (LFER) PET/CT system was used to follow the course of Mycobacterium tuberculosis (Mtb) infection in rhesus macaques. The animals were imaged before infection and every 2 weeks after infection beginning at 4 weeks for a maximum of eight PET-CT scans. [18F] FDG was injected intravenously (1 mCi/kg), and after 60 minutes incubation time a 20-min PET scan was acquired.

(C) Example PET-CT image from isotype control– (top) or PD-1 (bottom)–treated animals. (D) Fold change over week 4 value of total lung standardized uptake value (SUV) in isotype control (left) and PD-1–treated (right) animals.
By accepting you will be accessing a service provided by a third-party external to https://medisousa.com/