Innovation Delivered
The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule
Maryam Oroujeni1, Tianqi Xu1, Katherine Gagnon2,3, Sara S. Rinne3, Jan Weis4, Javad Garousi1, Ken G. Andersson5, John Löfblom5, Anna Orlova3,6, Vladimir Tolmachev1,6
An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with 11C-PBR28 and 18F-flumazenil PET Imaging
Krishna Kanta Ghosh1, Parasuraman Padmanabhan1,2, Chang-Tong Yang1,3,4, Zhimin Wang1, Mathangi Palanivel1 , Kian Chye Ng5, Jia Lu5, Jan Carlstedt-Duke6, Christer Halldin1,7 and Balázs Gulyás1,2,7
1 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore
2 Cognitive Neuroimaging Centre, Nanyang Technological University, Singapore
3 Department of Nuclear Medicine and Molecular Imaging, Radiological Sciences Division, Singapore General Hospital, Singapore
4 Duke-NUS Medical School, Singapore
5 DSO National Laboratories (Kent Ridge), Singapore;
6 President’s Office, Nanyang Technological University, Singapore;
7 Department of Clinical Neuroscience, Karolinska Institute, Sweden
https://doi.org/10.3390/ijms22020951
Summary
Traumatic brain injury (TBI) modelled by lateral fluid percussion-induction (LFPI) in rats is a widely used experimental rodent model to explore and understand the underlying cellular and molecular alterations in the brain caused by TBI in humans. The present study aims to investigate whether the two adioligands, 11C-PBR28 and 18F-flumazenil, are able to accurately quantify in vivo molecular-cellular changes in a rodent TBI-model for two different biochemical targets of the processes. As 11C-PBR28 is a radioligand of the 18 kD translocator protein (TSPO), the up-regulation of which is coupled to the level of neuroinflammation in the brain, and 18F-flumazenil is a radioligand for GABAA-benzodiazepine receptors, whose level capable of indicating at a high precision the neuronal loss that ensues in various brain disorders and injuries, the use of the two radioligands may reveal two critical features of TBI. An up-regulation in the 11C-PBR28 uptake triggered by the LFP in the injured (right) hemisphere was noted on day 14, while the uptake of 18F-flumazenil was down-regulated on day 14. When comparing the left (contralateral) and right (LFPI) hemispheres, the differences between the two in neuroinflammation were obvious. In vitro immunohistochemical analyses on the corpus callosum and hippocampal sections of the cerebrum were done to validate the results obtained in the PET imaging Results demonstrate a potential way to measure the molecular alterations in a rodent-based TBI model using PET imaging with 11C-PBR28 and 18F-flumazenil. These radioligands are promising options that can be eventually used in exploring the complex in vivo pharmacokinetics and delivery mechanisms of nanoparticles in TBI treatment.
Results from nanoScan PET/MRI
According to a standard LFP procedure, a combination of focal and diffuse injury was inflicted on the cerebral cortex and hippocampus of the right hemisphere of rats to create a TBI model for the study. The left hemisphere served as an internal control for the study. Following the LFP procedure on the brain’s right hemisphere of 12 Sprague Dawley rats, day 2 post operation 3D dynamic PET scans were performed using the nanoScan PET/MRI scanner. After injecting approximately 30±4MBq 11C-PBR28 or 18±4MBq 18F-flumazenil to the tail vein, dynamic 63min PET scan was performed with 11C-PBR28. The second PET radioligand 18F-flumazenil was injected 80min later, and the animals were scanned for a duration of 90min. The detailed time frames for the respective scan protocols were as follows: 8x15s, 4x30s, 2x1min, 2x2min, 4x5min, 3x10min for 11C-PBR28; 8x15s, 4x30s, 2x1min, 2x2min, 4x5min and 6x10min for 18F-flumazenil.
Results show:
- Compared to day 2 post-op, there is an increase in the uptake of 11C-PBR28 on day 14 due to the LFP in the right hemisphere (injured)
- 18F-flumazenil uptake was down-regulated on day 14, compared to day 2
- The time activity curves (TACs) of the whole brain also clearly demonstrate that there was a higher 11C-PBR28 and lower 18F-flumazenil uptake in day 14 as compared to day 2
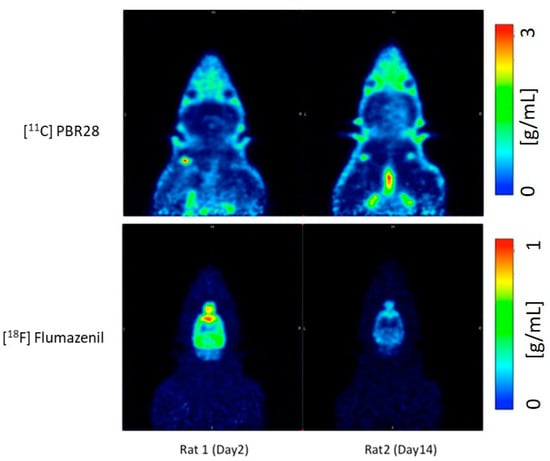
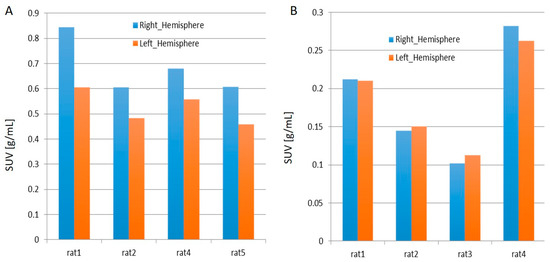
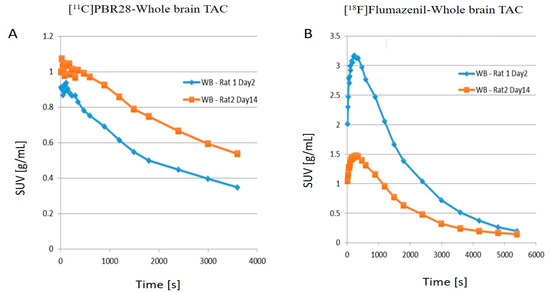
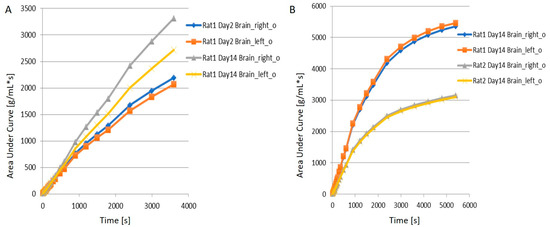
- When juxtaposing the right and left hemispheres using an area-under-the-curve (AUC) measure, the discrepancies between the two hemispheres for the 11C-PBR28 radiotracer were apparent. This is an indication that local increases in neuroinflammation due to the physical impact can be observed in the LFPI TBI rodent model. On the other hand, while 18F-flumazenil uptake is slightly higher in the left hemisphere, the lack of marked changes between the two hemispheres may reflect either the lack of neuron density alterations or the inappropriateness of the radioligand in indicating neuron density changes. This is the first LFPI TBI rat study to evaluate neuroinflammation and loss of neuronal density using two radioligands subsequently on the same day.
Para-chloro-2-[18F]fluoroethyl-etomidate: A promising new PET radiotracer for adrenocortical imaging
Isabella Silins1, Anders Sundin1, Patrik Nordeman2, Mahabuba Jahan2, Sergio Estrada2, Azita Monazzam3, Mark Lubberink1, Franklin Aigbirhio4, Per Hellman1, Gunnar Antoni2
1 Department of Surgical Sciences, Uppsala University
2 Medicinal Chemistry and Uppsala University
3 Medical Sciences at Uppsala University
4 Wolfson Brain Imaging Centre, University of Cambridge
https://www.medsci.org/v18p2187.htm
Summary
11C-Metomidate (11C-MTO) was developed as a PET radiotracer for adrenocortical tumours and has also been suggested for imaging in primary aldosteronism (PA). However, the use of 11C-MTO is somewhat hampered by considerable accumulation in the liver, which, because of its close proximity to the right adrenal gland, may obscure adrenal pathology and make PET measurements unreliable. Moreover, increased 11C-MTO uptake has been found in various liver lesions, such as adenoma, hepatocellular cancer and focal nodular hyperplasia, with the risk of false positive imaging results. Another disadventage of the tracer is that the clinical availability is restricted because of the short half-life of carbon-11 (T1/2= 20.4 min), which limits its use to PET centres with an in-house cyclotron and radiopharmacy.
The aim of this study was to evaluate the binding properties and in vivo behaviour of the previously published 18F-labeled (halflife 109.5min) etomidate analogue, para-chloro-2-18F-fluoroethyl etomidate; (18F-CETO), as an adrenal PET tracer. Comparative studies were also performed with 11C-MTO and with 18F-FETO, another adrenocortical imaging agent, which has not reached widespread clinical use, partial due to its two-stage radiosynthesis.
Autoradiography on human and cynomolgus monkey tissues show specific, high 18F-CETO uptake in normal adrenal cortex, as well as in human adrenocortical adenomas and adrenocortical carcinomas.
Following in vitro binding kinetic analysis and the evaluation of ex vivo biodistribution, in vivo imaging studies revealed high specificity of 18F-CETO accumulation in the adrenal cortex qualitatively surpassing those of 11C-MTO. Non-specific binding to the liver was significantly lower than that of 11C-MTO. 18F-CETO is a promising new PET tracer for imaging of adrenocortical disease and should be evaluated further in humans.
Results from nanoScan PET/MRI
18F-CETO imaging in rats and mice:
Eight female C57BL/6 mice and two male Sprague Dawley rats were were injected with of 18F-CETO (1.6±1MBq or 4-5MBq, respectively) and the 1h long dynamic PET imaging was started immediately. Four of the mice and one of the rats were co-injected with metomidate (1μmol/kg). The PET examination was followed by an MRI acquisition.
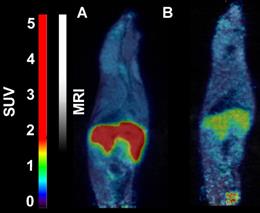
- 18F-CETO accumulated predominantly in the liver and in the adrenal glands, thus obfuscating the view of the adrenal glands in mice (Figure 1A: baseline; B: after blockage with metomidate)
- in contrast in rats the uptake was concentrated mainly in the adrenal glands with 120min p.i. peak adrenal uptake (Figure 2A: baseline; B: after blockage with metomidate)
18F-FETO imaging in rats:
Rats were were iv. injected with 4.0-4.8 MBq of 18F-FETO, one of the rats was given metomidate (1μmol/kg) and 1h long PET scan was started immediately.
- 18F-FETO in rats was also concentrated mainly in the adrenal glands. However, it was unable to block the uptake with metomidate, thus, re-evaluation of 18F-FETO has to be discontinued (Figure 2C: baseline; D: after blockage with metomidate)
Bispecific GRPR-Antagonistic Anti-PSMA/GRPR Heterodimer for PET and SPECT Diagnostic Imaging of Prostate Cancer
Bogdan Mitran1, Zohreh Varasteh1,2, Ayman Abouzayed1, Sara S. Rinne1, Emmi Puuvuori1, Maria De Rosa1,3, Mats Larhed1,4, Vladimir Tolmachev5, Anna Orlova1,4, Ulrika Rosenström1
1Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
2Department of Nuclear Medicine, Klinikum rechts der Isar der TUM, 81675 Munich, German
3Drug Discovery Unit, Ri.MED Foundation, 90133 Palermo, Italy
4Science for Life Laboratory, Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
5Department of Immunology, Genetics and Pathology, Uppsala University, 751 23 Uppsala, Sweden
https://doi.org/10.3390/cancers11091371
Whole body SPECT/CT scans of the mice bearing PC3-PIP xenografts injected with 111In-6 (400 kBq, 100 pmol/mouse) were performed using nanoScan SPECT/CT at 1, 3, and 24 h pi. Additionally, three mice were co-injected with either non-labeled PSMA-617 (1.5 nmol), non-labeled NOTA-PEG6-RM26 (1.5 nmol), or a combination of both, and imaged 1 h pi. CT scans were acquired at the following parameters: 50 keV, 670 μA, 480 projections, and 5 min acquisition time. SPECT scans were carried out using 111In energy windows (154 keV–188 keV; 221 keV–270 keV), a 256 × 256 matrix, and a 30 min acquisition time. The CT raw data were reconstructed using Nucline 2.03 Software. SPECT raw data were reconstructed using Tera-Tomo™ 3D SPECT.
PET/CT scans of the mice injected with 68Ga-6 (1.8 MBq, 100 pmol/mouse) were performed using nanoScan PET/MRI 3T at 1 h pi. To evaluate the in vivo specificity, one mouse was co-injected with a combination of non-labeled PSMA-617 (1.5 nmol) and non-labeled NOTA-PEG6-RM26 (1.5 nmol). CT acquisitions were performed as previously described using nanoScan SPECT/CT immediately after PET acquisition employing the same bed position. PET scans were performed for 30 min. PET data were reconstructed into a static image using the Tera-Tomo™ 3D reconstruction engine. CT data were reconstructed using Filter Back Projection. PET and CT files were fused and analyzed using Nucline 2.03 Software.
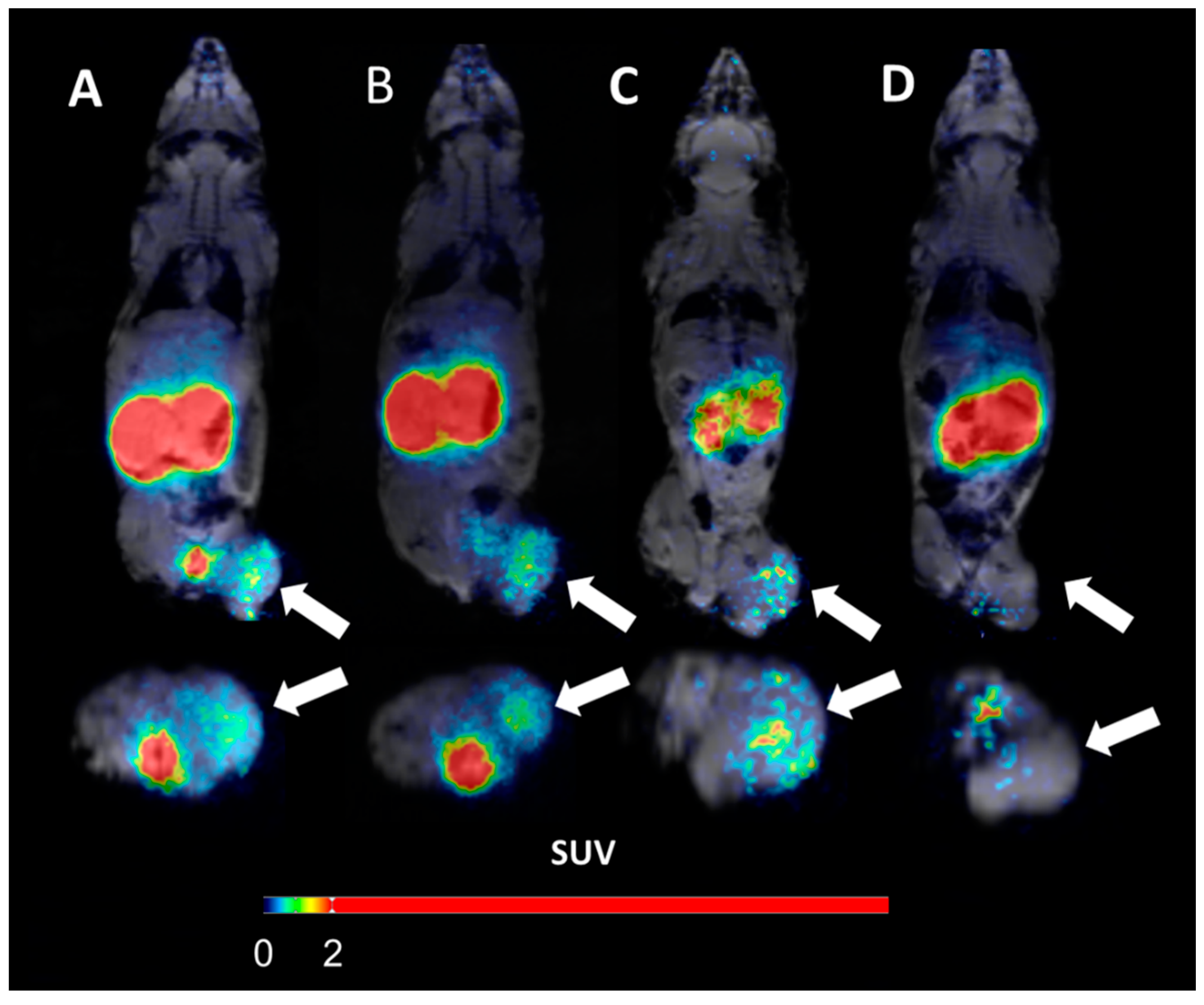
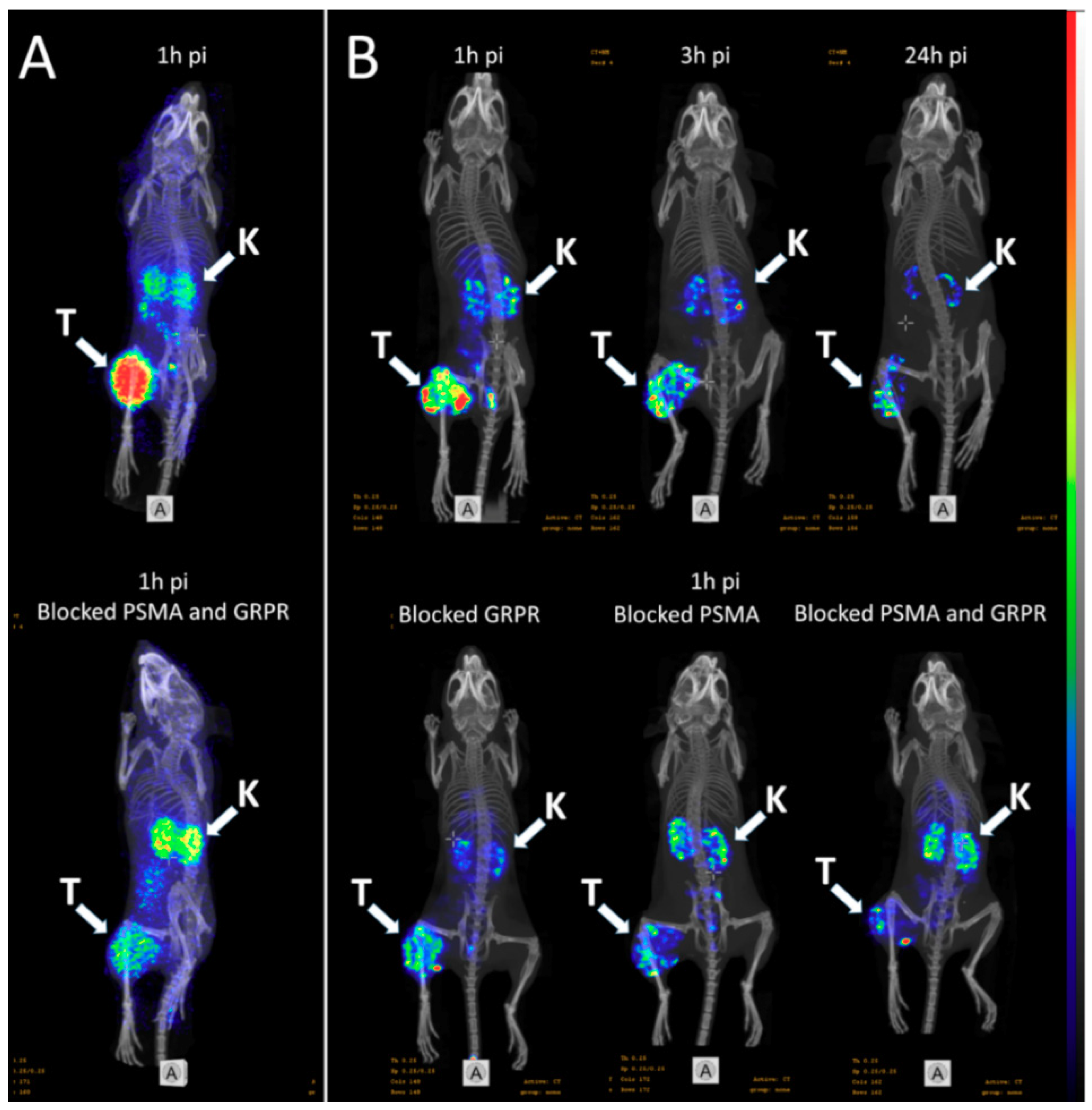
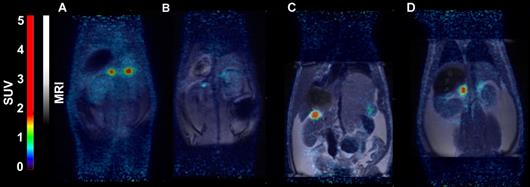
(A) micro positron emission tomography (microPET)/CT and (B) microSPECT/CT MIP images of PC3-PIP-xenografted mice (PSMA positive/GRPR positive) after an iv injection of 68Ga-6 and 111In-6. Blocked animals were co-injected with PSMA-617 for the blocking of PSMA and RM26 for the blocking of GRPR, or both.
- The imaging of PC3-PIP tumors using microPET/CT for 68Ga-6 (1 h pi) and microSPECT/CT for 111In-6 (1, 3, and 24 h pi) confirmed the ex vivo biodistribution data. Tumors were clearly visualized at all time-points, while weak activity accumulation was only observed in the kidneys among healthy organs. In agreement with ex vivo measurements, imaging contrast improved with time up to 3 h pi, despite the rapid washout of activity from tumors. The superior imaging contrast obtained for 111In-6 compared to 68Ga-6 was also in agreement with the biodistribution data. The imaging of mice after the co-injection of excess PSMA- and GRPR-targeting monomers corroborated with the ex vivo biodistribution pattern: partially decreased activity uptake in tumors, but increased activity uptake in kidneys, were exhibited in the case of PSMA blocking.
- MicroPET/CT and microSPECT/CT scans confirmed biodistribution data, suggesting that 68Ga-NOTA-DUPA-RM26 and 111In-NOTA-DUPA-RM26 are suitable candidates for the imaging of GRPR and PSMA expression in PCa shortly after administration.
Sigma-1 Receptor Positron Emission Tomography: A New Molecular Imaging Approach Using (S)-(−)-[18F]Fluspidine in Glioblastoma
Magali Toussaint 1, Winnie Deuther-Conrad 1, Mathias Kranz 1 2 3, Steffen Fischer 1, Friedrich-Alexander Ludwig 1, Tareq A Juratli 4, Marianne Patt 5, Bernhard Wünsch 6, Gabriele Schackert 4, Osama Sabri 5, Peter Brust 1
1 Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Institute of Radiopharmaceutical Cancer Research, Department of Neuroradiopharmaceuticals, Research site Leipzig, 04318 Leipzig, Germany.
2 PET Imaging Center, University Hospital of North Norway (UNN), 9009 Tromsø, Norway.
3 Nuclear Medicine and Radiation Biology Research Group, The Arctic University of Norway, 9009 Tromsø, Norway.
4 Department of Neurosurgery, Technische Universität Dresden (TUD), University Hospital Carl Gustav Carus, 01307 Dresden, Germany.
5 Department of Nuclear Medicine, University Hospital Leipzig, 04318 Leipzig, Germany.
6 Institute of Pharmaceutical and Medicinal Chemistry, University of Münster, 48149 Münster, Germany.
https://doi.org/10.3390/molecules25092170
Summary
Glioblastoma multiforme (GBM) is the most common primary tumors of the central nervous system. The survival rate for patients with GBM is dramatically low compared to patients with other brain tumor types. An important aspect contributing to this poor outcome is the genetic heterogeneity of GBM, which translates into heterogeneous expression patterns of potentially druggable targets. Hence the understanding of how spatiotemporal patterns evolve and change during pathogenesis would help to develop new targeted therapies, and biomarkers for treatment response.
The sigma-1 receptor (sig1R), an endoplasmic reticulum chaperone protein, is involved in signaling pathways assumed to control the proliferation of cancer cells and thus could serve as candidate for molecular characterization of GBM. The authors have used a selective sig1R ligand (S)-(−)-[18F]fluspidine to test this hypothesis with PET noninvasive molecular imaging.
In conclusion, the data obtained in the U87-MG mouse model of GBM along with the detection of sig1R in human GBM tissue for the first time by a PET radioligand, indicate not only the relevance of this target but also the suitability of (S)-(−)-[18F]fluspidine for sig1R-targeted cancer research and drug development.
Results from nanoScan PET/MRI
- Dynamic PET imaging showed that the uptake of (S)-(−)-[18F]fluspidine has higher retention in the tumor region compared to the CL at 60 min p.i., with SUVs of 0.38 and 0.28, respectively (Figure 4.)

Figure 4. PET/MR imaging of sig1R in mice with orthotopic xenograft of human GBM cells (U87-MG). Average time-activity curves after i.v. administration of (S)-(−)-[18F]fluspidine of the tumor (red dots) and the contralateral (black squares) regions of interest (n = 3). Statistical test: Student t-test, * p < 0.05.
- The early dynamic PET images between 2 and 9 min after injection show a heterogeneous uptake of (S)-(−)-[18F]fluspidine into the tumor (Figure 5D, upper panel), which may be caused by reduced blood supply to the tumor center. The PET image at later time points (45 to -60 min p.i.; Figure 5D, lower panel) pictures a more homogenous uptake of the tracer, along with a low slope, reflecting an accumulation.

Figure 5. (D) Representatives coronal PET/MR images of U87-MG tumor-bearing mouse after i.v. administration of (S)-(−)-[18F]fluspidine. The upper panel exhibits the distribution of (S)-(−)-[18F]fluspidine at early times p.i. (averaged time frames from 2 to 9 min), and the lower panel exhibits the distribution of (S)-(−)-[18F]fluspidine at later times (averaged time frames from 45 to 60 min). The regions-of-interest (ROIs) were delineated on the T2-weighted MR images and then applied on the PET data to generate the regional TACs.
Iodine‑124 PET quantification of organ‑specific delivery and expression of NIS‑encoding RNA
Matthias Miederer1, Stefanie Pektor1, Isabelle Miederer1, Nicole Bausbacher1, Isabell Sofia Keil2,
Hossam Hefesha3, Heinrich Haas3, Ugur Sahin2,3 and Mustafa Diken2,3
1 Department of Nuclear Medicine, University Medical Center of Johannes Gutenberg University, Mainz, Germany
2 TRON - Translational Oncology at the University Medical Center, Johannes Gutenberg University Mainz GmbH, Mainz, Germany
3 Biopharmaceutical New Technologies (BioNTech) SE, Mainz, Germany
https://doi.org/10.1186/s13550-021-00753-2
Summary
There has been increased interest in the development of mRNA-based vaccines for protection against various infectious diseases and also for cancer immunotherapies since lipid-based nanoparticles opened the possibility to deliver RNA to specific sites within the body, overcoming the limitation of rapid degradation in the bloodstream. In the present study, RNA-lipoplex nanoparticles were assembled by complexing sodium-iodide-symporter (NIS) coding mRNA with liposomes at different charge ratios. Two kinds of RNA-lipoplex systems were used: one system with net anionic charge mediating translation primarily within the spleen, and the other with net positive charge yielding translation primarily within the lungs. After in vitro analysis of the expression kinetics, mice were iv. injected with the mRNA-lipoplexes then 6h later with 124Iodine. Functional NIS protein translation was investigated by PET/MRI imaging. Results revealed rapid increase of 124Iodine uptake in the spleen or lung compared to control-RNA-lipoplexes (containing non-coding RNA) with minimal background in other organs except from thyroid, stomach and salivary gland (where NIS is physiologically expressed). The strong organ selectivity and high target-to-background acquisition of NIS-RNA lipoplexes indicate the feasibility of small animal PET/MRI to quantify organ-specific delivery of RNA.
Results from nanoScan PET/MRI
Female BALB/c mice were intravenously injected with RNA-lipoplexes containing 20μg NIS RNA. Six hours later 6.64±0.66MBq 124Iodine was injected intravenously. Three hours after 124Iodine injection, mice were anesthetized and static imaging was performed over 20min by nanoScan PET/MRI. Additionally, one animal per group was imaged dynamically for one hour.
- PET/MRI of anionic NIS-RNA lipoplexes showed a visually detectable increase of 124Iodine uptake in the spleen compared to control-RNA lipoplexes. Due to the high physiological NIS expression in the adjacent gastric wall, this increase was only visually clear with anatomical correlation by MRI. On PET imaging, spleen uptake appeared as an irregularity of the gastric wall which is not detected in control animals
- Lung uptake of NIS-RNA transported by cationic RNA-lipoplexes was depicted more clearly due to larger organ size and no adjacent physiological NIS uptake
- The quantified radioactivity from imaging matched well with the extent of uptake as measured in organs ex vivo, showing enhanced uptake of NIS-RNA and expression of functional NIS-protein in lung or spleen compared to the control RNA
- The uptake in lung was rapid and remained high over the first hour of dynamic acquisition

In vivo imaging with 18F-FDG- and 18F-Florbetaben-PET/MRI detects pathological changes in the brain of the commonly used 5XFAD mouse model of Alzheimer's Disease
Iodine-124 PET quantification of organ-specific delivery and expression of NIS-encoding RNA
Matthias Miederer1, Stefanie Pektor1, Isabelle Miederer1, Nicole Bausbacher1, Isabell Sofa Keil2, Hossam Hefesha3, Heinrich Haas3, Ugur Sahin2,3, Mustafa Diken2,3
1Department of Nuclear Medicine, University Medical Center of Johannes Gutenberg University, Mainz, Germany
2TRON - Translational Oncology at the University Medical Center, Johannes Gutenberg University Mainz gGmbH, Mainz, Germany
3Biopharmaceutical New Technologies (BioNTech) SE, Mainz, Germany
https://doi.org/10.1186/s13550-021-00753-2
Summary
RNA-based vaccination strategies tailoring immune response to specific reactions have become an important pillar for a broad range of applications. Recently, the use of lipid-based nanoparticles opened the possibility to deliver RNA to specific sites within the body, overcoming the limitation of rapid degradation in the bloodstream.
Nanoparticles show promising potency as delivery vehicles for a variety of molecules, leading to fundamentally new applications and therapeutic strategies. Due to their complex chemical composition and relevant interaction with plasma proteins, the pharmacokinetic properties and delivery properties of nanoparticles are variable and remain challenging to adapt for optimal conditions. Accomplishing precise RNA delivery to target tissue using nanoparticles would serve as a versatile platform that enables easy and transient expression of any protein in principal. RNA is currently already in use to selectively activate the immune system against specific target proteins for cancer therapy.
In the article, the authors have investigated whether small animal PET/MRI can be employed to image the biodistribution of RNA-encoded protein. For this purpose, a reporter RNA coding for the sodium-iodide-symporter (NIS) was assembled with liposomes at different charge ratios, and functional NIS protein translation was imaged and quantified in vivo and ex vivo by Iodine-124 PET upon intravenous administration in mice.
Results from the nanoScan PET/MRI
For the small animal imaging, the authors have used a nanoScan PET/MRI 1T, which provided a perfect option to follow the uptake of Iodine-124 not just in the thyroids, but also in the NIS-expressing tissues. Moreover, it could be accompanied by the help of MRI, to identify internal organs, like spleen.
Groups of n = 3 animals were intravenously injected with RNA-lipoplexes containing 20 µg NIS RNA. Six hours later 6.64 ± 0.66 MBq Iodine-124 was injected intravenously. Three hours after Iodine-124 injection, mice were anesthetized with 2% isoflurane and static imaging was performed over 20 min. For anatomic imaging MRI measurements (Material Map for coregistration of the PET scan; 3D Gradient Echo External Averaging (GRE-EXT), Multi Field of View (FOV); slice thickness: 0.6 mm; TE: 2 ms; TR: 15 ms; flip angle: 25 deg) were performed afterward. Additionally, one animal per group was imaged dynamically for one hour. PET data were reconstructed with Teratomo 3D (4 iterations, 6 subsets, voxel size 0.4 mm), co-registered to the MR and corrected for decay.
Figure 2. shows the PET/MRI of Iodine-124 distribution in vivo. (A) Coronal slices of PET/MRI fusion and volumes of interests (red) for spleen and lung are shown in representative animals. From left to right: targeting of spleen with non-coding RNA, targeting of spleen with NIS RNA, targeting of lung with non-coding RNA, targeting of lung with NIS-RNA. (B) Calculated organ uptake from the volumes of interests. Data are shown as mean + SD of n = 3 mice. (C) Representative in vivo bioluminescence images of Luc-RNA lipoplexes after targeting the spleen and lung. (D) Maximum intensity projections of PET images after application of lung-targeting NIS-RNA lipoplexes (right) in comparison with non-coding control (left). (E) Time activity curve of Iodine-124 uptake in the lung over 60 min immediately after Iodine-124 injection (6 h after administration of NIS-RNA lipoplexes targeting the lung).
- In this study, two RNA-lipoplex systems for systemic NIS-RNA delivery were compared by small animal PET/MRI of Iodine-124 uptake. One system with net anionic charge is known to mediate translation primarily within the spleen, and the other with net positive charge is known to yield translation primarily within the lungs.
- Tha authors have shown highly specific targeting, delivery and expression of RNA to spleen and lung by anionic and cationic RNA-lipoplex nanoparticles, respectively, through the use of the NIS reporter gene system and Iodine-124 uptake as imaged by PET/MRI. Combining NIS reporter gene imaging with in vivo small animal PET/MRI thus represents a powerful tool to monitor the distribution and extent of expression of RNA targeted specifically to any tissue over time.

Synthesis, in vitro and in vivo evaluation of 11C-O-methylated arylpiperazines as potential serotonin 1A (5-HT1A) receptor antagonist radiotracers
Vidya Narayanaswami, Junchao Tong, Ferdinando Fiorino, Beatrice Severino, Rosa Sparaco, Elisa Magli, Flavia Giordano, Peter M. Bloomfield, Jaya Prabhakaran3, J. John Mann, Neil Vasdev, Kenneth Dahl and S. Dileep Kumar
![]() https://doi.org/10.1186/s41181-020-00096-8
https://doi.org/10.1186/s41181-020-00096-8
Peter M. Bloomfield and his colleague, Junchao Tong, from Centre for Addiction and Mental Health (CAMH), Toronto, Ontario have used Mediso nanoScan PET/MR 3T for testing candidate serotonin receptor radioligands in this publication.
Summary
Clinical importance of 5-HT1A receptors in the pathogenesis of several psychiatric and neurodegenerative disorders has promoted development of carbon-11 and fluorine-18 labeled radiotracers for in vivo positron emission tomography (PET). The gold standard PET imaging agent limited its widespread use.
The purpose of the current study was to develop and characterize a radioligand with suitable characteristics for imaging 5-HT1A receptors in the brain. The authors have reported the in vitro pharmacological characterization, radiosynthesis and preliminary in vivo PET imaging of three new 5-HT1A receptor arylpiperazine based ligands in rats (DF-100 (1), DF-300 (2) and DF-400 (3)).
They concluded DF-400 represents a promising O-methylated lead candidate which if subjected to structural alterations, may either lead to improved selectivity for 5-HT1A receptors or may assist in the development of the first PET radioligand for α1-adrenergic receptors.
Results from nanoScan PET/MRI 3T
- Dynamic PET studies in rats demonstrated negligible brain uptake of [11C] DF100 (1) and [11C] DF-300 (2). In contrast, significant brain uptake of [11C] DF400 (3) was observed.

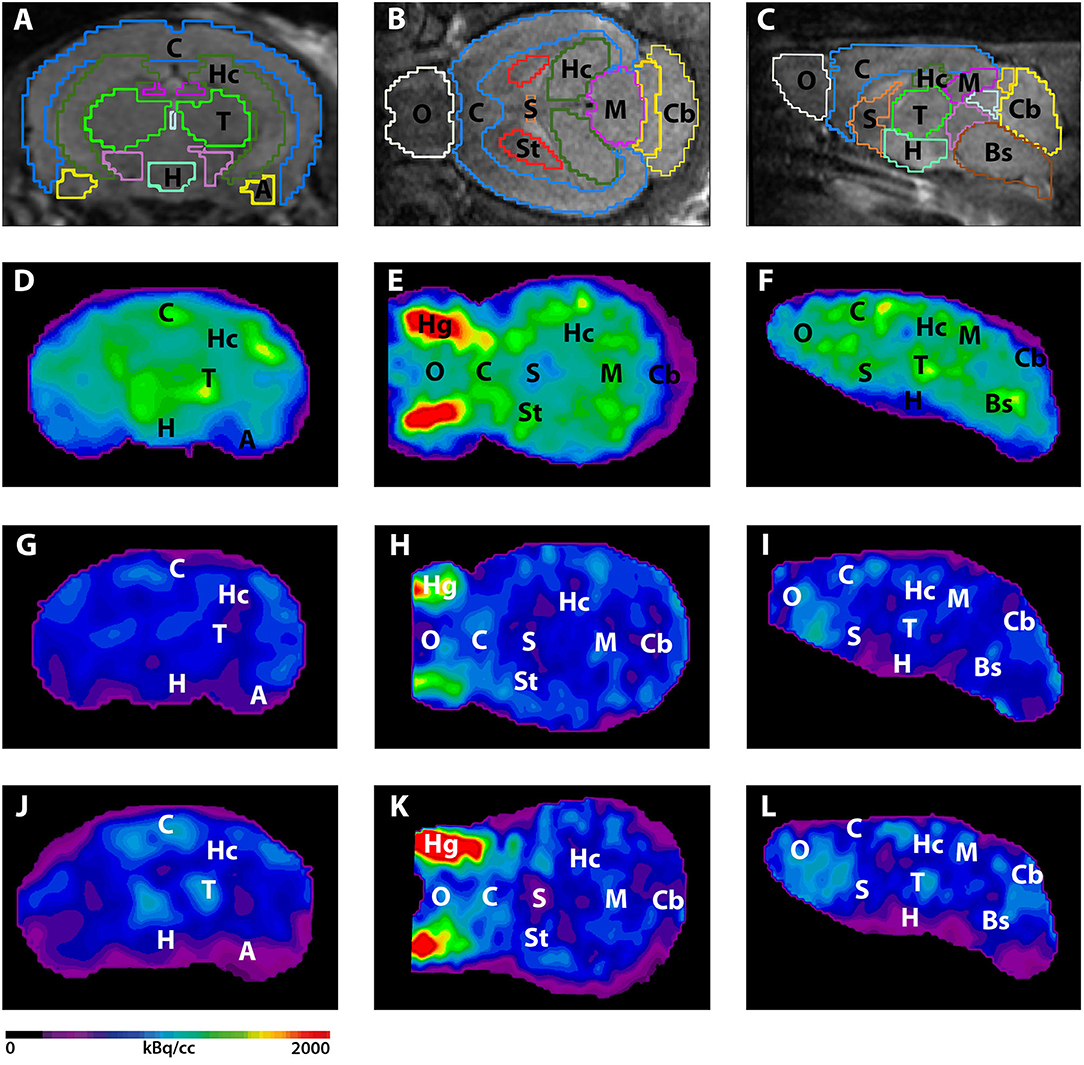
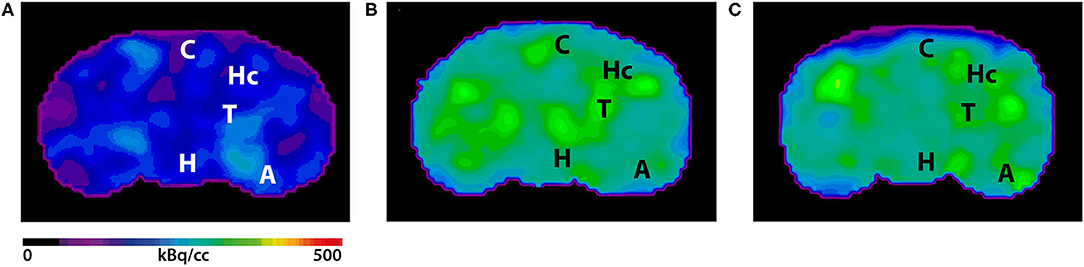
Fig. 2 Uptake of [11C]3 (a); [11C]2 (b) and [11C]1 (c) in rat brain. Shown are TACs averaged for left and right brain (A: n = 3; B and C: n = 1) in SUV and summed (0–60 min) PET images in coronal, transverse and sagittal planes, respectively, through the thalamus. The spatially co-registered MR images (2D fast spin echo) show left-half ROIs including thalamus (blue), anterior cingulate cortex (red), hippocampus (green) and cerebellum (magenta) for the corresponding color-coded TACs
- Nevertheless, DF-400 displayed significant off-target binding attributed to α1-adrenergic receptors based on regional distribution (thalamus>hippocampus) and blocking studies

Fig. 2 Blocking of the uptake of [11C]3 in rat brain by WAY-100635 (a) and prazosin (b). Shown are TACs, averaged for left and right brain, (n = 1; solid: baseline; dashed: blocking) in SUV and summed (0–60 min) PET images in coronal, transverse and sagittal planes, respectively, through the thalamus at baseline and under blocking conditions. The three depicted left-half ROIs include thalamus (orange), hippocampus (red) and cerebellum (magenta) for the corresponding color-coded TACs
PET imaging of P2X7R in the experimental autoimmune encephalomyelitis model of multiple sclerosis using [11C]SMW139
https://doi.org/10.1186/s12974-020-01962-7
Summary
Neuroinflammation plays a central role in a variety of pathologies affecting the central nervous system (CNS), such as multiple sclerosis (MS), Alzheimer’s and Parkinson’s disease. Microglia are major contributor in disease’s pathogenesis, although the exact role of microglia and their activation status during the disease process is not understood exactly.
In this article the process of neuroinflammation has been studied in Lewis rats with experimental autoimmune encephalomyelitis (EAE), an animal model for MS.
Mediso nanoScan PET/CT and nanoScan PET/MRI were used for non-invasive imaging of the activation status of microglia and the ability to identify a pro- or anti-inflammatory environment.
The authors have used a C-11 isotope labelled purinergic receptor (P2X7R) ligand ([11C]SMW139) for tracing microglial activity. They assessed the tracer’s potential for imaging neuroinflammation and its specific binding to P2X7R. They also matched the molecular imaging result with autoradiography and immunohistochemistry.
The authors have shown that [11C]SMW139 is a promising PET tracer for imaging neuroinflammation and evaluating the dynamics of pro-inflammatory microglia in the brain.
Selected results from nanoScan PET/MRI and nanoScan PET/CT
- They evaluated the uptake of [11C]SMW139 at the peak of inflammation and compared it to the uptake in the recovery phase.

Fig 1. Sagittal PET images extracted from the static reconstruction of the 5–45 min frame and showing [11C]SMW139 uptake in the brain and spinal cord (arrows) of severe-relapsing (a), severe acute (b). Arrow heads are showing [11C]SMW139 uptake in a brain draining lymph node

Fig 2. Sagittal PET images extracted from the static reconstruction of the 5–45 min frame and showing [11C]SMW139 uptake in the brain and spinal cord (arrows) of relapsing EAE rat (peak of the disease (e), relapse (f), recovery (g)) and non-relapsing rat ( of the disease (h), no-relapse (i), recovery (j)). S.C. spinal cord, CB cerebellum, B.S. brain stem. Data are expressed as percent injected dose per milliliter (%ID/mL)
- They validated the specificity of [11C]SMW139 tracer binding to the EAE tissue

Fig 3. Correlation between uptake of [11C]SMW139 tracer in the brain of the EAE animal and the ex vivo immunostaining for IBA-1 and ED-1; Transversal (A) and sagittal (D) PET image section showing the uptake of the [11C]SMW139 in the brain. The dotted purple circles or rectangles mark the area with the highest uptake. The green and red spots in the brain indicate a high accumulation of [11C]SMW139; Immunostaining with IBA-1 (B, E) and CD68 (C, F) of the respective brain region post PET imaging showing high microglia activation in the same region where the high uptake of [11C]SMW139 was observed by PET imaging.
A study by researchers at Huntsman Cancer Institute (HCI, Salt Lake City, Utah) has been recently published in the journal ‘Nature Medicine’. The paper proposes a new therapeutic approach for patients suffering from pancreatic cancer that may be effective to treat the disease. It involves a combination drug therapy and has been studied in vitro, in vivo also in a human volunteer.
As the new treatment combines two drugs that are both registered for use by the Food and Drug Administration, the clinical trial (‘THREAD’) is now open at HCI soon to be followed by other US sties.
The novel method involves targeting two physiological process at the same time. Previous studies have focused on only one process and shown to be ineffective. One process is an impact of a mutation in a gene called ‘KRAS’ that sends constant signals that promote cells divisions resulting in uncontrolled tumour growth. Another process, autophagy, is a cell-level recycling of cells including pancreatic cancer cells.
The new HCI study used mouse models to investigate a drug response of combining the two drugs simultaneously and applied advanced imaging techniques (nanoScan PET/MRI, supplied by Mediso USA, Boston and nanoScan SPECT/CT, Mediso, Hungary installed in HCI at the Center for Quantitative Cancer Imaging) to show the strong drug response.
“We were able to observe that the combination of these two drugs — which, when used individually, don’t have much of an impact on the disease — appears to have a very potent impact on the growth of pancreatic cancer,” says McMahon, PhD, a cancer researcher at HCI and Professor of Dermatology. “We have observed this in the lab in petri dishes, then in mouse models, and now in a pancreatic cancer patient on a compassionate use basis. Indeed, we proceeded from a petri dish to a patient in less than two years — a timeline that is rarely seen in medical science.”

Preclinical imaging. Mice were anesthestized and injected by approximately 0.5 mCi of [18 F]-fluorodeoxyglucose (FDG). CT imaging was performed using a NanoScan SPECT/CT scanner followed by PET and MRI imaging using a NanoScan PET/MRI scanner (Mediso Medical Imaging, Budapest). The animal remained anesthetized and immoblized in a common MultiCell animal chamber to provide intrinsic spatial co-registration of CT, MRI, and PET images. T1-weighted Gradient Echo (GRE) images and T2-weighted 2D Fast Spin Echo (FSE) images were acquired prior to initiating a 20-minute PET emission scan at 60 minutes post-injection of FDG. (Figures from this paper are publicly accessible at: https://www.nature.com/articles/s41591-019-0367-9)
At Mediso USA, we are proudly supporting researchers with state-of-the-art advanced imaging techniques in their efforts to shorten time from bench to clinic. Huntsman Cancer Institute Becomes has been the First Mediso Preclinical Imaging Center of Excellence in North America since 2015. They are the first USA site site using the nanoScan 3Tesla PET/MRI.
In the first published article from MSKCC (Carney, B. et al. Non-invasive PET Imaging of PARP1 Expression in Glioblastoma Models. Mol Imaging Biol 1–7 (2015)), using the nanoScan PET/MRI (1T) small animal imaging system, in vivo whole body PET/MRI imaging of [18F]PARPi in orthotopic brain tumor-bearing mice is referenced.
[18F]PARPi is a selective PARP1 imaging agent that can be used to visualize glioblastoma in xenograft and orthotopic mouse models with high precision and good signal/noise ratios offering new opportunities to non-invasively image tumor growth and monitor interventions.
Figure 6 in the article shows coronal views of contrast-enhanced MRI, [18F]PARPi PET images, and fused PET/MRI of orthotopic U251 MG tumor-bearing mice. In the top row the mouse receivied only [18F]PARPi, in the bottom row the mouse receivied [18F]PARPi after a 500-fold excess of olaparib.

The animals were injected with 200 µCi of [18F]-PARPi and a 20 minutes static PET scan was acquired 2 hours post injection. 200 µL of diluted gadopentate dimegumine in saline solution was administered intravenously one minute prior to MRI acquisition. Tumor regions were identified on anatomic images acquired using a post-contrast T-weighted spin-echo (SE) acquisition. The co-localization of [18F]PARPi and tumor in PET/MRI studies was confirmed by ex vivo autoradiography. In PET/MRI fusion images, accumulation in the tumor was co-aligned with the orthotopic tumor on MRI. In mice receiving an injection of olaparib ahead of the radiotracer, the [18F]PARPi tumor uptake was negligible.
It's important to note that no further or manual co-registration was required at all as the PET/MRI studies performed on the nanoSCan PET/MRI are co-registered by nature due to the common gantry and automated acquisition system. The very same images are displayed in the viewer when the dual-modality study is loaded from the DICOM server after reconstruction. This gives scientists confidence when evaluating multi-modal data; changing animal physiology and data obtained at different times won't distort the findings.
Added a new article to the Selected Review Articles section of our Literature page:
De Jong, Marion, Jeroen Essers, and Wytske M. van Weerden. “Imaging Preclinical Tumour Models: Improving Translational Power.” Nature Reviews Cancer 14, no. 7 (July 2014): 481–93. doi:10.1038/nrc3751.
It's interesting to note that the very first NanoSPECT/CT installation took place in the main author, Professor Marion De Jong's lab back in January 2006. Apparently it's confirmed again that life is cyclical - the first academic nanoScan SPECT/MRI installation also took place at Erasmus MC earlier this Fall.
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature advance online publication, (2014) Published online 15 October 2014
It’s rare when an Nature article is directly relied on in vivo imaging experiment. The ‘Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors’ article was published online in Nature on 15 October 2014. Dr. Peter Brust, Professor at Helmholtz-Zentrum Dresden - Rossendorf, Institute of Radiopharmaceutical Cancer Research, Research Site Leipzig participated in the design and data analysis of the PET/MRI studies published in article. In his very recent talk at the EANM 2014 Mediso Preclinical User Workshop he gave the insight for the audience that molecular biology and conventional laboratory test results were actually crowned by the results of the in vivo imaging experiments performed with our nanoScan PET/MRI.
Introduction
Brown adipose tissue (BAT) is specialized in energy expenditure, making it a potential target for anti-obesity therapies. However current BAT therapies based on cold exposure or B-adrenergic agonists are clinically not feasible, therefore alternative strategies has to be explored for developing new therapy possibilities. The researchers showed that adenosine activates human and murine brown adipocytes at low nanomolar concentrations. and induces browning of WAT. In the light of the world-wide obesity pandemic, activators of BAT may be potential drug targets for anti-obesity therapies and as shown here, adenosine is a previously unappreciated activator of BAT.
Adenosine role in BAT activating
Adenosine is released in BAT during stimulation of sympathetic nerves as well as from brown adipocytes. Pharmacological blockade or genetic loss of A receptors in mice caused a decrease in BAT-dependent thermogenesis, whereas treatment with A2A agonists significantly increases energy expenditure. Moreover, pharmacological stimulation of A2A receptors or injection of lentiviral vectors expressing the A receptor into white fat induced brown-like cells—so-called beige adipocytes. Importantly, mice fed a high-fat diet and treated with an A agonist are leaner with improved glucose tolerance.
The detailed analysis required a suitable animal model that mimics the response of human BAT to adenosine. The in vivo imaging results validated the original hypothesis that adenosine receptors' agonist ligands really activate the activities of brown adipose tissue.
 In vivo PET/MRI studies
In vivo PET/MRI studies
The PET/MRI studies of BAT activation were performed on nanoScan PET/MRI (Mediso Medical Imaging Systems, Hungary) using male anaesthetized C57BL/6 WTmice. Subcutaneous injection of vehicle, noradrenaline or PSB-0777 (the A2A agonist) (both 1 mg per kg body weight) was performed one minute before intraperitoneal injection of 14.7+/-0.4 MBq of [18F]FDG. The activity in the interscapular BAT region at 75 min post injection was expressed as mean standardized uptake value.
 Stimulation with noradrenaline or AAA agonist caused a significantly higher uptake of [18F]FDG compared to vehicle treatment into murine BAT as measured with positron emission tomography/magnetic resonance imaging.
Stimulation with noradrenaline or AAA agonist caused a significantly higher uptake of [18F]FDG compared to vehicle treatment into murine BAT as measured with positron emission tomography/magnetic resonance imaging.
Closing remarks
Taken together, the results demonstrated that adenosine–A2A signalling plays an unexpected physiological role in sympathetic BAT activation and protects mice from high-fat diet-induced obesity. Those findings reveal new possibilities for developing novel obesity therapies. The featured Mediso nanoScan fully integrated PET/MRI system is completely mature, reliable system and installed at fifteen sites currently, including Kayvan R. Keshari, PhD lab at Memorial Sloan Kettering Cancer Center in New York City, NY.
Development of dual-modality, aluminium hydroxide stabilised magnetic nanoparticles probes is published in the Biomaterials 2014 July issue. The main author of the article titled ‘Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging’ [1] is Dr. Xianjin Cui, member of Philip Blower’s group at King's College London, Division of Imaging Sciences and Biomedical Engineering. The article is a collaboration between researchers from King’s College London (UK), Nottingham University (UK), Aston University (UK), CROmed Ltd. (Hungary). This is an open access article. Download the Article in PDF, Appendix A in Word.
Superparamagnetic nanoparticles
Superparamagnetic nanoparticles (NPs) have been intensively investigated due to their potential applications in biosensors, targeted drug develivery, MRI and localised hyperthermia induction. The problem with these nanoparticles is that they tend to aggregate to minimize the surface energy. Bio-applications require colloidal stability and dispersibility in water and biological environments. There are several methods described in the literature to obtain stable colloids of magnetic nanoparticles. A simple approach is presented in the article to stabilise magnetic nanoparticles by coating them with an Al(OH)3 layer via a hydrolysis process for conjugation. The use of an inorganic shell material introduces stability, functionality (nanoparticle recognised by the macrophage-monocytic system) and water-solubility. The materials, general characterisation, synthesis and radiolabelling are described in the article.
in vivo PET/MR imaging
What is interesting for our blog is that for in vivo PET/MR imaging of the agents on mice were performed on the integrated nanoScan preclinical PET/MRI imaging system installed at the Nanobiotechnology & In Vivo Imaging Center, Semmelweis University in Budapest, Hungary.
The total injected F-18 activity was 0.95 MBq (25.7 microCi). PET scanning was started immediately after injection and continued for 120 min. Acquisition took place in 1–5 coincidence mode with 5 ns coincidence window, 400–600 keV energy window. MR scanning was performed immediately after PET. A 3D expectation maximisation (3D EM) PET reconstruction algorithm (Mediso Tera-Tomo™) was applied to produce PET images including corrections for attenuation and scatter, dead time, decay and randoms. After 8 iterations the reconstruction stopped resulting in images with 0.1 mm voxel size and time frames of 8 × 15 min. The images of the two modalities were fused automatically.
The PET/MRI fused image is presented in the Appendix A. of the article. The injected activity was only 0.95 MBq (25.7 microCi) and the PET images show only 15 minutes of acquisition!
 In vivo PET/MRI images of a normal young C57BL/6 mouse using 18F radiolabelled 3: (a) whole body PET image showing distribution of 18F 30 minutes post injection (maximum intensity projection, mice in prone position); (b) PET/MRI fused image (coronal section, 0-15 minutes); (c) PET/MRI fused image (coronal section, 105-120 minutes); (d) MR image prior to the injection of NPs, and (e) MR image post the injection of NPs, showing a darkening contrast at lung and live area. Due to the unstable Al(OH)3 shell, 18F-fluoride radioactivity was released from magnetic NPs 3 within 15 minutes and localised in bone.
In vivo PET/MRI images of a normal young C57BL/6 mouse using 18F radiolabelled 3: (a) whole body PET image showing distribution of 18F 30 minutes post injection (maximum intensity projection, mice in prone position); (b) PET/MRI fused image (coronal section, 0-15 minutes); (c) PET/MRI fused image (coronal section, 105-120 minutes); (d) MR image prior to the injection of NPs, and (e) MR image post the injection of NPs, showing a darkening contrast at lung and live area. Due to the unstable Al(OH)3 shell, 18F-fluoride radioactivity was released from magnetic NPs 3 within 15 minutes and localised in bone.
The reconstruction features the TeraTomo algorithm's latest version which will be available for all our sites this autumn. In our opinion it is hard to get better bone images nowadays with PET for such a low injected activity than it’s featured in this article. Funnily enough noone intended to make bone images as this is a proof that the radiolabel went off from the nanoparticles and trapped in bones of the mouse. Remember, this is not a F-18 flouride bone scan! The ‘grainy’ PET image isn't the result of any regularization issue – this represents the real uneven flour uptake in the bones. You can notice the anatomical features of the knee joint – the patella, condyles of femur can be distinguished as well!
Read more about the integrated, automated small animal whole-body PET/MRI system.
[1] Cui, X. et al. Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging. Biomaterials doi:10.1016/j.biomaterials.2014.04.004
 This article was published in discovered, The HZDR Research Magazine (Issue 02.2013, December 2013/January 2014, ISSN: 2194-5713; PDF 2.2MB)
This article was published in discovered, The HZDR Research Magazine (Issue 02.2013, December 2013/January 2014, ISSN: 2194-5713; PDF 2.2MB)
Six white CD-1 mice are scurrying through the litter in their cage, climbing the metal bars, nibbling away at the pellets they are being fed, and snuggling with each other. What they don't yet know is they're about to participate in a pivotal study. One that will save lives - those of mice and, one day, of men. As part of his dissertation, Mathias Kranz, Ph.D. student at the HZDR Research Site Leipzig, is currently investigating the degree of radioactivity that builds up within the bodies of mice whenever radioactive probes - called radiotracers - are used, and identifying in which organs specifically it accumulates. Eventually, these data will be extrapolated to the human magnitude. Radiotracers are chemical compounds that include a radioactive element of some sort, which can help scientists observe metabolic processes in living organisms.
Specifically, in the case of the Leipzig project, we're talking about the two radiotracers [18F]fluspidine and [18F]flubatine - both of them molecules containing the radionuclide 18F (fluorine). They're supposed to ultimately find their way into the diagnostics of cancers and neurodegenerative diseases like Alzheimer's. Key is their ability to imitate properties of various endogenous structures.
Before a radioactive probe is ready for use in the hospital setting, its efficacy and safety must first be documented in living organisms.
Once injected into the human body, they bind with high affinity to certain targets - in the case of the "PET sugar" [18F]FDG, which is also used at the Leipzig site, highly metabolically active tissues like tumors. The emitted radiation from the radioactive molecules can be captured and subsequently analyzed using positron emission tomography (PET). However, before a radioactive tracer can be introduced into the hospital setting, its efficacy and safety to the living organism must first be confirmed. This is a prerequisite imposed by the German Federal Office for Radiation Protection (BfS) and the Federal Institute for Drugs and Medical Devices (BfarM). This multistep procedure starts with work on mice and occasionally pigs and ultimately leads to research conducted on healthy human subjects. Here, the HZDR scientists are receiving support from their colleagues at the Clinic for Nuclear Medicine at Leipzig University Hospital.
Leipzig as reference site
As of spring 2013, when operations by experienced colleagues at the HZDR main site Dresden first commenced, Germany's first-ever commercial full-body PET/MRI for small animals opened in Leipzig - one of only a few worldwide. The HZDR is the reference site for Hungarian manufacturer Mediso (Budapest) - which brings with it a number of obvious benefits: "There are still a handful of delayed-onset childhood illnesses but whenever we do report any problem, help typically arrives within a matter of hours," Mathias Kranz explains. The 27-year-old fellow, who holds a master's in engineering, studied biomedical technology at Ilmenau University of Technology, and has been working at the HZDR Institute of Radiopharmaceutical Cancer Research for about a year now. He is thrilled with the new device: "Not only does it allow us to obtain information about metabolic processes that are happening inside the body, it also yields high-resolution three-dimensional images that document the exact location and distribution of soft tissues." especially when it comes to brain imaging, MR devices yield far better results than conventional PET and computer tomography (CT) combinations.
The mice remain safe
"Without these methods, we would need to dissect the animal subjects, remove individual organs, and then measure them in order to determine the degree of radioactivity that has accumulated in the body following injection of the radiotracer. What's interesting is not only the current dose rate but also how it changes over the course of minutes and hours, which helps determine the organ dose. Thanks to PET/MRI, we're able to conduct even long-term studies using the same exact mouse," Mathias Kranz explains. In the case of other methods, one laboratory animal has to be sacrificed each time a single measurement is obtained.
During examination, the mice are lying on a heated animal bed, their breathing monitored with the help of a pressure sensor. The radioactively labeled substance is injected into the tail vein. The mice are fully anesthetized and won't remember anything afterwards. On a screen, Mathias Kranz is now examining a black and grey image showing the inside of the mouse's body. Red, yellow, and blue spots are lighting up in certain body regions. "Red means these are sites where there is a high degree of radioactivity, in other words that a lot of our substance was deposited in these places," the young scientist explains. At first glance, the liver, kidneys, and bladder are easily recognized - organs, which are actively involved in the substance's elimination from the body.
After the experiments are done, Mathias Kranz calculates the expected effective human dose. This serves as a risk-assessment at the time of introducing the probes into the clinical setting. Based on their results, the researchers have filed for approval of a study with the BfS for use of their newly developed radiotracers (+)-[18F]flubatine and (S)-(-)-[18F]fluspidine in humans. The scientists are working closely with their colleagues at Leipzig University Hospital, Department of Nuclear Medicine, on these projects. The projected start date is early 2014.
Back in May 2013, I gave a talk titled "The Motivations and Systems for High Content In-vivo Tomographic Imaging in
Drug Discovery" at the 6th Imaging in Drug Discovery & Development Conference in Boston. Mediso USA was the Silver Sponsor for the event.
According to GTC this is "the only imaging conference that brings together high-level/influential leaders with decision-making authority from the pharmaceutical industry, academia, and government to share their knowledge and expertise in drug discovery and development". Needles to say, the sessions were indeed interesting, with an interesting mix from academia, government, pharma and imaging companies.
Session topics included:
- Advantages and Challenges of Available Imaging Modalities
- Translational Imaging Applications: Preclinical to Clinical
- Imaging Applications Across Multiple Therapeutic Areas
- Molecular Imaging and Diagnostic Approaches and Capabilities
- High Content Imaging, Quantitative Imaging and Modeling Capabilities
The November issue of the was published today in the Genetic Engineering & Biotechnology News, with the Feature Article: Raising the Bar in Preclinical Imaging written by MaryAnn Labant. The article is based on presentations given at the May GTC Imaging in Drug Discovery and Development Conference.
Please find below our related section from the second page of the online article.
Integrated Imaging Systems
Preclinical PET scanners with an integrated microCT have substantially improved the anatomical registration of PET predominately to the skeleton, yet little progress has been made in soft tissue contrast, even with the use of a CT contrast agent.
 Integrated PET/MRI or SPECT/MRI systems offer many benefits. MRI uses no radiation, offers better soft tissue contrast, and provides molecular readouts. To date, preclinical PET imaging combined with MRI has been performed using two independent systems and a bespoke co-registration algorithm to fuse the images.
Integrated PET/MRI or SPECT/MRI systems offer many benefits. MRI uses no radiation, offers better soft tissue contrast, and provides molecular readouts. To date, preclinical PET imaging combined with MRI has been performed using two independent systems and a bespoke co-registration algorithm to fuse the images.
Mediso recently commercialized the first serially produced, fully integrated, automated PET/MRI system, the nanoScan PET/MRI, and a fully integrated, automated SPECT/MRI system, the nanoScan SPECT/MRI. Single systems enable use of the same imaging technology, imaging protocol, and biomarkers for small to large subjects.
According to Illes J. Muller, managing partner, preclinical PET/MRI and SPECT/MRI allow combination of radionuclide biomarkers with an MRI contrast agent on a routine basis, an attractive prospect for evaluating new drugs for oncology, neurology, and cardiovascular disease. Now, physiological/metabolic readouts can be combined with high-resolution, soft-tissue contrast as well as dynamic functional perfusion imaging.
 In addition, SPECT provides the ability to perform multi-isotope imaging, probing two or more molecular pathways simultaneously by detecting isotopes with different emission energies, and has no physical limits in resolution. SPECT/MRI technology is less expensive. The labeling is easier, and no on-site cyclotron is required.
In addition, SPECT provides the ability to perform multi-isotope imaging, probing two or more molecular pathways simultaneously by detecting isotopes with different emission energies, and has no physical limits in resolution. SPECT/MRI technology is less expensive. The labeling is easier, and no on-site cyclotron is required.
A potential major application for multimodal emission tomography combined with MRI is quantitative 3D imaging of tumor heterogeneity. To assess the spatial distribution of a given PET or SPECT biomarker within a tumor requires ultra-high resolution and high sensitivity and corrections for tumor perfusion. MRI is able to differentiate between healthy and dead tumor tissue for tumor response evaluation.
Note: This was the related section from the article, with links added to the relevant pages of Mediso USA website.
Blog Image
The blog image shows a Mouse Tumor Heterogeneity Study performed with nanoScan PET/MRI. The mouse was injected with 3 MBq Ga68-DOTA-TATE and imaged for 15 minutes at 60-75 min post injection.
Starting from April 29 the National Institutes of Health (NIH) is now accepting applications for the Shared Instrumentation Grant (SIG) Program and the High End Instrument Grant (HEI) Program.
The submission deadline is May 29, 2015.
The objective of these programs is to make available to institutions expensive, commercially available research systems that cost at least $50,000 (SIG Program) or at least $600,000 (HEI Program). The maximum award is $600,000 for the SIG program and $2,000,000 for the HEI Program.
The instruments can only be justified on a shared-use basis and that are needed for NIH-supported projects in basic, translational or clinical areas of biomedical/behavioral research (description from nih.gov). The SIG Program provides funds to purchase or upgrade a single item of expensive, specialized, commercially available instrument or an integrated instrumentation system to be used for research purposes only. To promote cost effectiveness, to encourage optimal sharing among individual investigators, research groups and departments, and to foster a collaborative multidisciplinary environment, the instrument should be integrated in a centralized core facility, whenever possible.
We, Mediso USA provide support to submit a successful instrumentation grant and we are committed to supporting you throughout the grant process. Please contact us for more details.
External Links
- Shared Instrumentation Grant (SIG) Program (S10): http://grants.nih.gov/grants/guide/pa-files/PAR-15-088.html
- High-End Instrumentation (HEI) Grant Program (S10): http://grants.nih.gov/grants/guide/pa-files/PAR-15-118.html
Coast to Coast
We were thrilled this year to announce that Mediso USA reached a major milestone with the establishment of its tenth preclinical nanoScan imaging system in North America. We are looking back this holiday season with so much appreciation for all of you in making this possible.

Teaming Up
It was a great honor to have our first site in North America designated as a Center of Excellence for Preclinical Imaging. Many thanks to the Center for Quantitative Cancer Imaging team at the Huntsman Cancer Institute (HCI), part of the University of Utah Health Care system in Salt Lake City. We look forward to continuing our partnership into the New Year.
State of the Art
With its nanoScan PET/MRI(3T) installations dotting the globe, Mediso accepts only the best in imaging performance. As such, the nanoScan PET/MRI(3T) system features a 3T translational MR field strength combined with exceptional PET performance in a compact cryogen-free and low fringe field design, guarantying low running costs and an easy-to-use workflow.
Up and Coming
Our team is also looking forward to a major advance on our horizon. We are proud to say that 2016 will feature our first MultiScan LFER 150 PET/CT installation in the U.S. The large bore in-vivo imaging system is tuned for translational research, capable of whole-body NHP imaging. Time to plan those F220 replacements!
Last month, in October a new review article titled Preclinical Imaging: an Essential Ally in Modern Biosciences on preclinical imaging technologies was published in the Molecular Diagnosis & Therapy journal. The journal provides insights into the latest molecular diagnostic and pharmacogenomic techniques and their use in personalized medicine.
Cunha, Lídia, Ildiko Horvath, Sara Ferreira, Joana Lemos, Pedro Costa, Domingos Vieira, Dániel S. Veres, et al. 2013. “Preclinical Imaging: An Essential Ally in Modern Biosciences.” Molecular Diagnosis & Therapy: 1–21. doi:10.1007/s40291-013-0062-3.
The find out that actually what is small-animal or preclinical imaging, P. Zanzonico from MSKCC has provided a good definition, stating that 'it constitutes a way of assessing biological structures and function in vivo by noninvasive means, allowing the collection of quantitative information, both in health and disease states' [1].
The main role of preclinical imaging is to deliver translational answers for serious health-related problems of the growing and aging world population. Small animal models have to represent a bridge between discoveries at the molecular level and clinical implementation in diagnostics or therapeutics. Small animal imaging is being used in a wide variety of lines of research, especially in infection, inflammation, oncology, cardiology, and neurosciences.
The article summarizes the general properties of diagnostic imaging modalities and reviews them one-by-one including Positron emission tomography (PET), Single photon emission computed tomography (SPECT), Optical imaging (OI), Computed tomography (CT), Magnetic resonance imaging (MRI) and Ultrasound (US) and their related instrumentation of these modalities in small animal imaging. A separate and well detailed section is dedicated to the comparison of micro-SPECT and micro-PET. The general parameters are summarized in a large table listing imaging characteristics (spatial resolution, sensitivity, penetration depth, temporal resolution), related costs, probe types, major advantages, disadvantages and their application areas.
There are inherent limitations to each imaging modality - this has brought commercial multi-modality systems 10+ years ago to the market. Multimodal combination has enabled some of the most important limitations of each imaging modality to be overcome when used alone. The considerations are explained in the tenth sections of the article.
It's an honor to see multi-modality images of PET/MRI and SPECT/MRI acquired by our nanoScan imagers in the article.
A SPECT/MRI application was selected as the image of this blog post. The image shows transverse slices of SPECT and MRI images of a mouse brain. SPECT was acquired using a specific agent for cortical benzodiazepine receptors (123I-NNC13-82431). The lack of anatomical information of SPECT acquisition is complemented with the information provided by MRI, in which the eyes, the olfactory bulbs and the first and second ventricles are shown. The multimodality SPECT/MRI image provides information about functional benzodiazepine receptors from SPECT allied to good soft tissue contrast from the MRI.
Abstract of the Article
Translational research is changing the practice of modern medicine and the way in which health problems are approached and solved. The use of small-animal models in basic and preclinical sciences is a major keystone for these kinds of research and development strategies, representing a bridge between discoveries at the molecular level and clinical implementation in diagnostics and/or therapeutics. The development of high-resolution in vivo imaging technologies provides a unique opportunity for studying disease in real time, in a quantitative way, at the molecular level, along with the ability to repeatedly and non-invasively monitor disease progression or response to treatment. The greatest advantages of preclinical imaging techniques include the reduction of biological variability and the opportunity to acquire, in continuity, an impressive amount of unique information (without interfering with the biological process under study) in distinct forms, repeated or modulated as needed, along with the substantial reduction in the number of animals required for a particular study, fully complying with 3R (Replacement, Reduction and Refinement) policies. The most suitable modalities for small-animal in vivo imaging applications are based on nuclear medicine techniques (essentially, positron emission tomography [PET] and single photon emission computed tomography [SPECT]), optical imaging (OI), computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy imaging (MRSI), and ultrasound. Each modality has intrinsic advantages and limitations. More recently, aiming to overcome the inherent limitations of each imaging modality, multimodality devices designed to provide complementary information upon the pathophysiological process under study have gained popularity. The combination of high-resolution modalities, like micro-CT or micro-MRI, with highly sensitive techniques providing functional information, such as micro-PET or micro-SPECT, will continue to broaden the horizons of research in such key areas as infection, oncology, cardiology, and neurology, contributing not only to the understanding of the underlying mechanisms of disease, but also providing efficient and unique tools for evaluating new chemical entities and candidate drugs. The added value of small-animal imaging techniques has driven their increasing use by pharmaceutical companies, contract research organizations, and research institutions.
[1] Zanzonico P. Noninvasive imaging for supporting basic research. In: Kiessling F, Pichler BJ, editors. Small animal imaging—basics and practical guide. Heidelberg: Springer; 2011. p. 3–16. (Springer; Google Books)