Innovation Delivered
Myocardial perfusion recovery induced by an a-calcitonin gene-related peptide analogue
Simon Bentsen, MD1, Anette Sams, PhD2, Philip Hasbak, MD, DMSc1, Lars Edvinsson, MD, PhD, DMSc2, Andreas Kjaer, MD, PhD, DMSc1, Rasmus S. Ripa, DMSc1
1Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark
2Department of Clinical Experimental Research, Glostrup Research Institute, Glostrup University Hospital, Glostrup, Denmark
https://doi.org/10.1007/s12350-021-02678-8
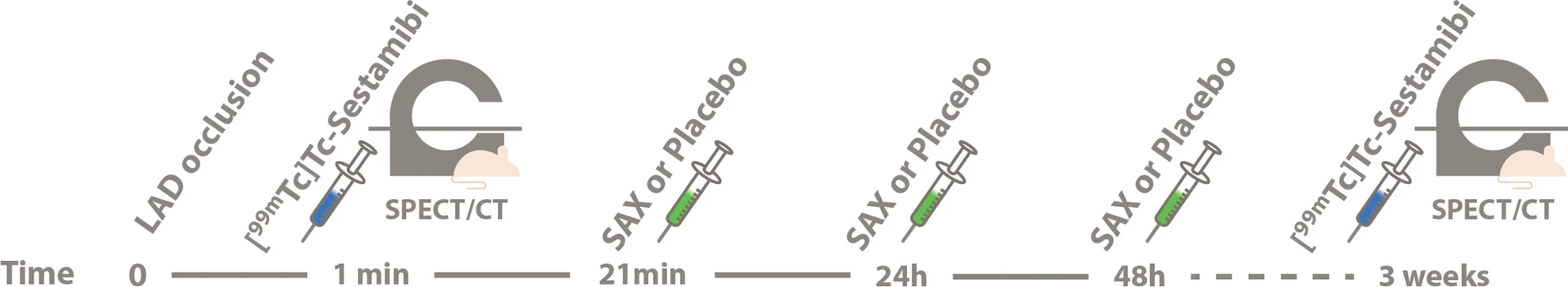
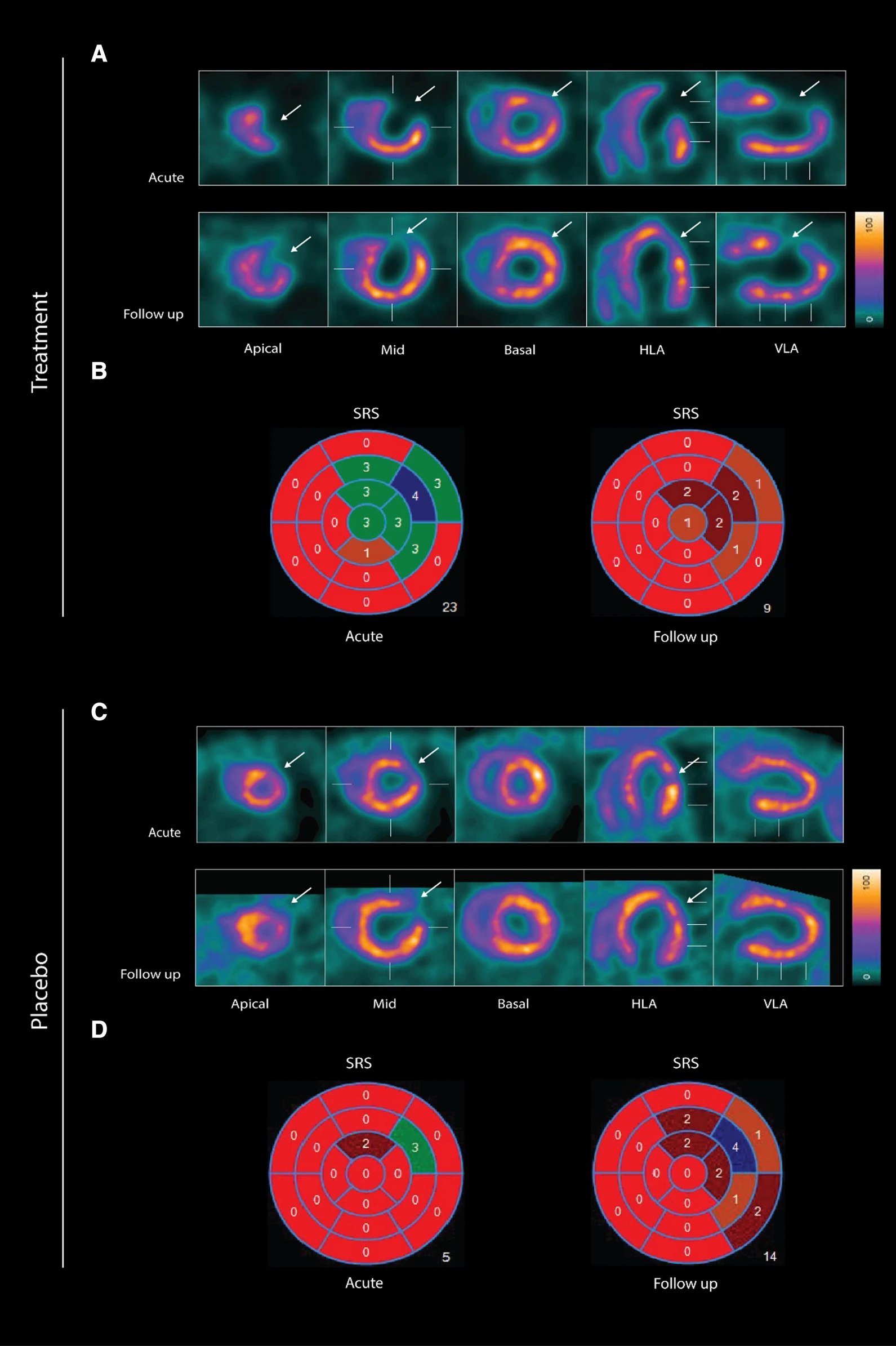
wo representative examples of rats with chronic LAD occlusion from the SPECT/CT scans. 4DM software was used to analyze [99mTc]Tc-sestamibi SPECT/CT. Top panels (A and B) show a SAX-treated rat. Bottom panels (C and D) show a placebo-treated rat.(A) Large perfusion defect in the anterior and apical wall in the acute scan, with a smaller perfusion defect at follow-up scan after SAX treatment (white arrow).(B) The perfusion defects from panel A in a 17-segment polar map.(C) Medium perfusion defect in the anterior wall at the acute scan, and a more severe perfusion defect at follow-up after placebo treatment (red arrow).(D) The perfusion defects from panel C in a 17-segment polar map. HLA horizontal long axis, VLA vertical long axis, SRS summed rest score.
- The results show that an analogue of CGRP that induces both coronary and peripheral vasodilation significantly improves myocardial perfusion recovery after experimental myocardial infarction in rats. There was no significant difference in overall survival between the two groups suggesting that SAX does not have a damaging effect on the animals or the myocardium.
- In conclusion, the CGRP analogue, SAX, seems to have a cardioprotective effect on a rat model of myocardial infarction, by improving the perfusion recovery after a chronic occlusion of the coronary artery.
Simultaneous Visualization of 161Tb- and 177Lu-Labeled Somatostatin Analogues Using Dual-Isotope SPECT Imaging
Francesca Borgna1, Patrick Barritt1, Pascal V. Grundler1, Zeynep Talip1, Susan Cohrs1, Jan Rijn Zeevaart2, Ulli Köster3, Roger Schibli1,4, Nicholas P. van der Meulen1,5 and Cristina Müller1,4
1 Center for Radiopharmaceutical Sciences, Paul Scherrer Institute, 5232 Villigen-PSI, Switzerland
2 Radiochemistry, South African Nuclear Energy Corporation (Necsa), Brits 0240, South Africa
3 Institut Laue-Langevin, 38042 Grenoble, France
4 Department of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland
5 Laboratory of Radiochemistry, Paul Scherrer Institute, 5232 Villigen-PSI, Switzerland
https://doi.org/10.3390/pharmaceutics13040536
Summary
177Lu (half-life 6.7d) is currently the most often applied radiometal for therapeutic purposes, as it has a particulate emission (β− or Auger electron) for effecting therapy and emits several accompanying γ-photons of 208 keV (11%) and 113 keV (6.4%), which are used for diagnostic evaluation and dosimetry.
161Tb is a more recently introduced radiolanthanide for therapeutic applications. 161Tb decays with a half-life of 6.89 days to stable 161Dy, while emitting β¯-particles (Eβ͞av = 154 keV) suitable for therapeutic purposes and γ-radiation (Eγ = 49 keV, I = 17.0%; Eγ = 75 keV, I = 10.2%) useful for SPECT imaging. 161Tb also emits a substantial number of low-energy conversion and Auger electrons, which makes this radionuclide exceptionally interesting for the treatment of disseminated cancers with multiple metastases ranging from a single cell (diameter: ~10μm) to micro cell clusters (diameter: < 1mm). Monte Carlo simulations assessed the dose delivered to 10μm spheres revealed a 3.5-fold increased value when using 161Tb as compared to 177Lu. In larger tumors (diameter > 10mm), the emitted electron energy from 161Tb and 177Lu respectively is almost entirely absorbed, resulting in a 1.3-fold higher absorbed electron energy fraction per decay for 161Tb compared to 177Lu, making 161Tb more potent than 177Lu.
The aim of the present study was to use dual-isotope SPECT imaging in order to demonstrate that 161Tb and 177Lu are interchangeable without compromising the pharmacokinetic profile of the radiopharmaceutical.
After in vitro characterization, 161Tb- and 177Lu-labeled somatostatin (SST) analogues DOTATOC (agonist) and DOTA-LM3 (antagonist) were injected to AR42J tumor-bearing nude mice. In vivo disptribution profiles were investigatd by dual-isotope SPECT/CT imaging. Results revealed identical pharmacokinetic profiles of the two peptides, irrespective of whether it was labeled with 161Tb or 177Lu. Moreover, the visualization of the sub-organ distribution confirmed similar behavior of 161Tb- and 177Lu-labeled SST analogues. These and previous findings suggest that any future (pre)clinical studies with 161Tb can be based on preclinical data obtained with its 177Lu-labeled counterpart. This will allow the focusing of future investigations directly on the therapeutic efficacy of 161Tb, which is likely to be superior to the effect obtained with 177Lu.
Results from nanoSPECT/CT
Five-week-old female CD1 nude mice were subcutaneously inoculated with AR42J tumor cells (5x106 cells in 100µl PBS). The scans were performed 10–14 days after tumor cell inoculation when the tumor size reached a volume of ~250mm3.
Mice were i.v. injected with a mixture of 161Tb-DOTATOC (~15MBq) and 177Lu-DOTATOC (~15MBq) or a mixture of 161Tb-DOTA-LM3 and 177Lu-DOTA-LM3 (~30MBq) at a 161Tb/177Lu activity ratio of 1:1. For specificity test, blocking studies were performed under the same experimental conditions; however, in this case, an excess of unlabeled DOTATOC or DOTA-LM3 was added to the injection solution. SPECT/CT scans were acquired 2h, 4h, and 24h after injection of the radiopeptides using the dual-isotope SPECT acquisition protocol with a frame time of 60s resulting in a scan time of 45min.
For the SPECT/CT scan, simultaneous acquisition of counts stemming from 161Tb and 177Lu, respectively, was performed by the selection of distinct energy windows for the two radionuclides. The two energy windows chosen for 161Tb were set at 47.7keV±10%, which enabled the detection of X-rays and γ-rays (46.0keV, 48.9keV and 52.0keV), and at 74.6keV±10%, enabling the detection of the γ-rays at 74.6keV. For 177Lu, the windows were set at 112.9keV±10% and 208.4±10% to detect the γ-rays. Prior to animal studies, phantom scans were carried out in order to verify of the dual-isotope SPECT imaging protocol using Eppendorf vials filled with 161Tb or 177Lu or both: analysis revealed no interference between 161Tb and 177Lu in the acquired scans; each radionuclide was visualized independently of the other with high accuracy.
Analysis of the SPECT/CT images revealed:
- Equal in vivo distribution of simultaneously injected 161Tb-DOTATOC and 177Lu-DOTATOC. The same observation was made for 161Tb-DOTA-LM3 and 177Lu-DOTA-LM3. Images reconstructed using the energies of either radiolanthanide (red-to-yellow scale and green-to-yellow scale for 161Tb and 177Lu, respectively), provided the distribution for each radiopeptide separately in the same mouse.
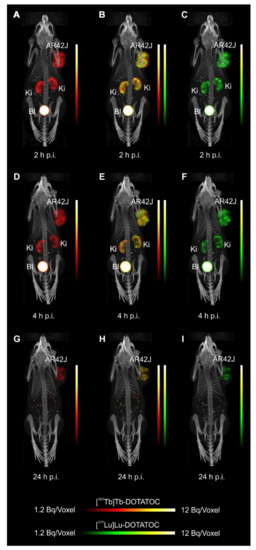
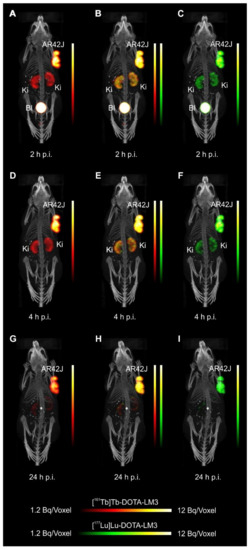
- Experiments performed by co-injection of excess unlabeled peptide resulted in SSTR blockade and, hence, accumulation of the radiopeptides in AR42J tumors was not observed. These additional studies proved that the uptake of the SST analogues in AR42J tumor xenografts was SSTR-specific.
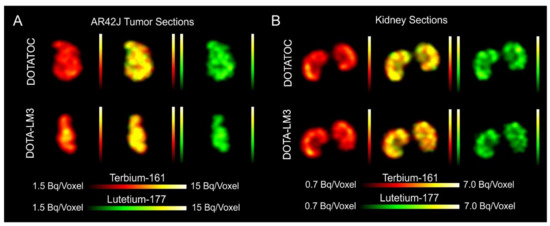
- Quantification of the accumulated activity in AR42J tumors and kidneys confirmed equal distribution of the 161Tb- and 177Lu-labeled counterparts. This was the ultimate proof that the chosen radiolanthanide did not have an impact on the tissue distribution profile of the radiopeptides.
- The SPECT/CT images showed activity accumulation in the AR42J xenografts, which was higher for the antagonist than for the agonist. In agreement with quantitative data from biodistribution studies, the activity was efficiently cleared through the kidneys over time and almost entirely excreted after 24h. Due to the favorable uptake of radiolabeled DOTA-LM3 in the tumor tissue, the tumor-to-kidney ratio was higher as compared to the ratio obtained after injection of radiolabeled DOTATOC.
- Dual-isotope SPECT image sections enabled, for the first time, visualization of the 161Tb- and 177Lu-labeled peptide distribution at a sub-organ level in the same animal. Most important to note is that the pattern of activity distribution in tumors and kidneys was the same, irrespective of whether 161Tb or 177Lu was used. The uptake in the tumor was quite homogenous, which can be ascribed to the well-vascularized AR42J xenograft. Accumulation of activity in the kidneys was more prominent in the cortex where the megalin-mediated reabsorption of radiopeptides occurs, and where various SSTR subtypes are known to be expressed
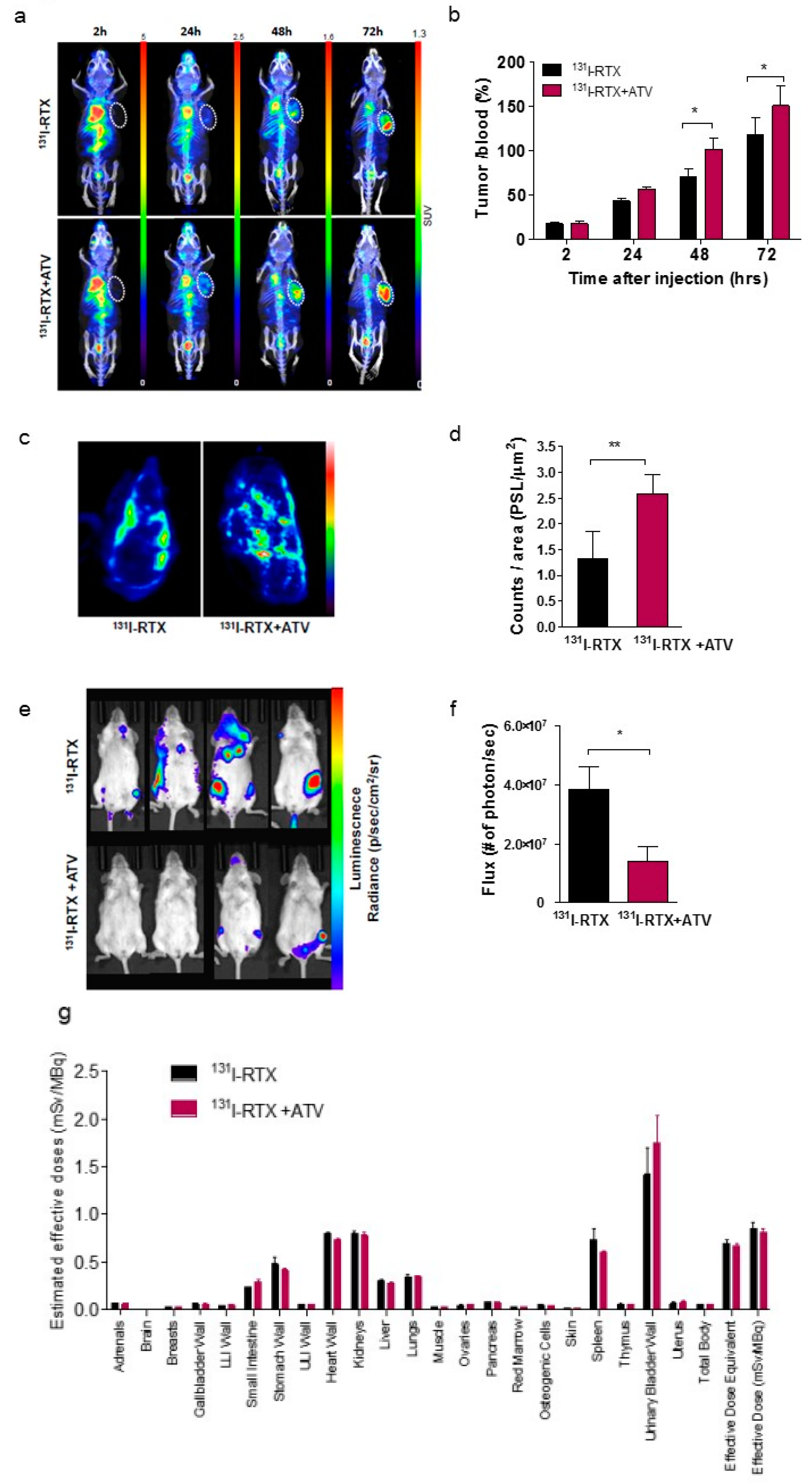
Inhibition of HIF-1α by Atorvastatin During 131I-RTX Therapy in Burkitt’s Lymphoma Model
Eun-Ho Kim1,2, Hae Young Ko3,4, A Ram Yu5, Hyeongi Kim3, Javeria Zaheer3,6, Hyun Ji Kang3,6, Young-Cheol Lim3, Kyung Deuk Cho3, Hyun-Yoo Joo1, Min Kyoung Kang5, Jae Jun Lee5, Seung-Sook Lee7, Hye Jin Kang8, Sang Moo Lim3,9, Jin Su Kim3,6
SPECT imaging evaluation of 111indium-chelated cetuximab for diagnosing EGFR-positive tumor in an HCT-15-induced colorectal xenograft
Bin-Bin Shiha, Yi-Fang Changb, Chun-Chia Chengb, Hao-Jhih Yangc, Kang-Wei Changc, Ai-Sheng Hoa, Hua-Ching Lind, Chun Yeha, Chun-Chao Change,f
a Division of Gastroenterology, Cheng Hsin General Hospital, Taipei, Taiwan, ROC
b Hematology and Oncology, Mackay Memorial Hospital, Taipei, Taiwan, ROC
c Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan, ROC
d Division of Proctology, Cheng Hsin General Hospital, Taipei, Taiwan, ROC
e Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei, Taiwan, ROC
f Division of Gastroenterology and Hepatology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, ROC
http://dx.doi.org/10.1016/j.jcma.2017.02.010
Summary
Colorectal cancer (CRC) occurs with high incidence worldwide, but is usually diagnosed in late stage with metastasis by the conventional methods. Epidermal growth factor receptor (EGFR) is overexpressed in 97% of CRC cells, serving a promising diagnostic candidate. In the present study, Cetuximab, an anti-EGFR monoclonal antibody was conjugated with an isotope chelator, diethylene triamine penta acetic acid (DTPA), labeled with 111indium (111In) and injected to tumor bearing mice. Biological distrubution was investigated by SPECT/CT imaging.
Results revealed that 111In-Cetuximab accumulated in the both small (50mm3) and large (250mm3) tumors, whereas the ratio of tumor to muscle in the large tumor was 7.5-fold. The biodistribution data indicated that the 111In-cetuximab bound to tumor specifically that was higher than that in other organs. Consequently, 111In-cetuximab is suggested to be suitable for early diagnosis and prognostic monitor of EGFR-positive CRC in further clinical practice.
Results from nanoSPECT/CT
- The tumor of the 111In-Cetuximab group was apparently observed both in 24h and 48h and higher than that in the 111In group.
- 111In-Cetuximab majorly accumulated in liver and tumor, otherwise, 111In accumulated only in the kidney.
- The tumor to muscle ratio of 111In-Cetuximab was measured 7.5-fold, which was higher than that of 111In group measured as 3.1-fold, indicating that 111In-cetuximab specifically bound to EGFR-positive tumors as a reliable diagnosing agent.
- The result also indicated that 111In labeled with Cetuximab through chelator DTPA was easily excreted out the mice better than free 111In, suggesting that this labeling method may not lead to accumulation of 111In metal in mice.

Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1
Anzhelika Vorobyeva1,2,† , Elena Konovalova3,†, Tianqi Xu1, Alexey Schulga2,3, Mohamed Altai1, Javad Garousi1, Sara S. Rinne4, Anna Orlova2,4,5, Vladimir Tolmachev1,2 and Sergey Deyev2,3,6,7
1 Department of Immunology, Genetics and Pathology, Uppsala University, 751 85 Uppsala, Sweden
2 Research Centrum for Oncotheranostics, Research School of Chemistry and Applied Biomedical Sciences, Tomsk Polytechnic University, Tomsk, Russia
3 Molecular Immunology Laboratory, Shemyakin & Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia
4 Department of Medicinal Chemistry, Uppsala University, Uppsala, Sweden
5 Science for Life Laboratory, Uppsala University, Uppsala, Sweden
6 Bio-Nanophotonic Lab, Institute of Engineering Physics for Biomedicine (PhysBio), National Research Nuclear University ‘MEPhI’, Moscow, Russia
7 Center of Biomedical Engineering, Sechenov University, Moscow, Russia
† A.V. and E.K. contributed equally
https://doi.org/10.3390/ijms21093310
Summary
Up to 85% of ovarian cancer patients are diagnosed only at advanced stages, when cancer has already spread through the body. The present study aimed to find a more efficient way for diagnosis and treatment using mouse xenograft models.
As epithelial cell adhesion molecule (EpCAM) is overexpressed in 55%–75% of ovarian carcinomas (OC), it might be a promising target. Designed ankyrin repeats protein (DARPin) Ec1 binds to EpCAM with subnanomolar affinity. In the present study, DARPin Ec1 was labeled with 125I using N-succinimidyl-para-iodobenzoate (PIB) and injected to mice bearing SKOV-3 or OVCAR-3 xenografts. In vitro experiments showed highly specific binding to ovarian carcinoma cells, moreover, slow internalization, which is essential for in vivo imaging a few hours after injection. In vivo biodistribution analyses of SPECT/CT images suggest that EpCAM on ovarian cancer xenografts is sufficiently accessible to permit DARPin-mediated delivery of cytotoxic payload.
Results from nanoScan SPECT/CT
For establishment of xenografts, 107 of SKOV-3 and OVCAR-3 cells or 5x106 Ramos cells (EpCAM-negative lymphoma xenografts served as specificity control) in 100µl of media were subcutaneously injected in the right hind leg of female Balb/c nu/nu mice. The experiments in mice bearing SKOV-3 and Ramos xenografts were performed 2–3 weeks after implantation. The experiments in mice bearing OVCAR-3 xenografts were performed 7 weeks after implantation.
Mice were injected with 125I-PIB-Ec1 (20µg, 1.2MBq for SKOV-3, and 6µg, 2.8MBq for OVCAR-3), SPECT/CT images were acquired 6h pi time later for 20min. with nanoScan SPECT/CT.
- In vitro studies revealed specific binding to SKOV-3 and OVCAR-3 cells; rapid binding and slow dissociation and internalization
- SPECT/CT imaging demonstrated that radiolabeled 125I-PIB-Ec1 provided clear visualization of both EpCAM-expressing xenografts. In vivo biodistribution is characterized by high tumor-to-organ ratio, the only organ with noticeable activity were kidneys.

Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging
Falco Reissig1, David Bauer1,2, Martin Ullrich1, Martin Kreller1, Jens Pietzsch1,2, Constantin Mamat1,2, Klaus Kopka1,2, Hans-Jürgen Pietzsch1, Martin Walther1
1Helmholtz-Zentrum Dresden-Rossendorf, Institut für Radiopharmazeutische Krebsforschung, Bautzner Landstraße 400, D-01328 Dresden, Germany
2Fakultät Chemie und Lebensmittelchemie, Technische Universität Dresden, D-01062 Dresden, Germany
https://doi.org/10.3390/ph13100272
Summary
Barium-131 is a single photon emission computed tomography (SPECT)-compatible radionuclide for nuclear medicine and a promising diagnostic match for Radium-223/-224. In the early 1970s, Barium-131 has been thoroughly investigated as a potential bone targeting radiotracer, but no substantial benefits have been mentioned, comparing it to other already applicable radiotracers like [18F]F− (t½ = 110 min) and 99mTc-labeled (t½ = 6.0 h) bisphosphonates. However, as part of current approaches to the therapy of bone cancer and bone metastases, this radionuclide has its significance in modern times. Barium-131 possesses the suitable half-life of 11.5 d, thereby making it highly beneficial for potential diagnostic use in nuclear medicine. Due to the similar chemistry and pharmacological properties of the elements Barium and Radium, Barium-131 is particularly feasible as a diagnostic match to the therapeutic α-emitters Radium-223 and Radium-224. In the work presented here, the authors aimed to establish a simple but sufficient procedure for the production and purification of n.c.a. Barium-131 using the TR-FLEX cyclotron (ACSI), starting from a cheap Cesium Chloride target with natural monoisotopically occurring Cesium followed by 27.5 MeV proton bombardment. Moreover, the in-house produced Barium-131 was used for first labeling studies with the chelator macropa, for initial in vivo-related phantom studies and, last but not least, small animal imaging trials with [131Ba]Ba(NO3)2 and 131Ba-labeled macropa in healthy mice.
Results from the nanoScan SPECT/CT
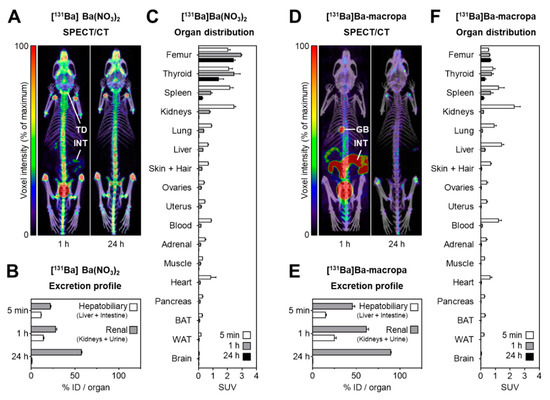
For the small animal imaging, the authors have used a nanoScan SPECT/CT, to follow the biodistribution with the different Barium-131 tracers.
SPECT/CT imaging in mice was performed at 1 h and 24 h after i.v. injection of [131Ba]Ba(NO3)2 (6.2 MBq in 0.2 mL of 0.01 M HNO3, pH 6, Am = 420 GBq/µmol, n.c.a.), or 131Ba-labeled macropa (6.7 MBq in 0.2 mL of 0.1 M ammonium acetate, pH 6, Am = 83 MBq/µmol) with a frame time of 60 s (total scan time: 1.5 h), respectively. The acquisition was performed using a standard aperture for mouse imaging (APT62) consisting of four M3 multi-pinhole collimators providing a 30 × 30 mm transaxial field of view (FOV). Projection data were reconstructed using the Tera-Tomo™ 3D high dynamic range algorithm (resolution: 128; iterations: 48; subset size: 4), applying corrections for decay, scatter, and attenuation.
Figure 8. shows the distribution of [131Ba]Ba(NO3)2 and [131Ba]Ba-macropa in mice. (A) SPECT/CT fusion images of [131Ba]Ba(NO3)2 in a mouse 1 h and 24 h after injection; (B,C) excretion profile and organ distribution of [131Ba]Ba(NO3)2 in mice 5 min, 1 h, and 24 h after injection (n = 4); (D) SPECT/CT fusion images of [131Ba]Ba-macropa in a mouse 1 h and 24 h after injection; (E,F) excretion profile and organ distribution of [131Ba]Ba-macropa in mice; 5 min, 1 h, and 24 h after injection (n = 4); (BAT) brown adipose tissue; (GB) gall bladder *; (ID) initial dose; (INT) intestine; (TD) thyroid/parathyroid *; (WAT) white adipose tissue (* activity in these organs was not measured separately).
- The author have shown for the first time the in vivo biodistribution behavior of 131Ba-labeled macropa in comparison with free [131Ba]Ba2+ by means of small animal SPECT/CT.
- Biodistribution studies revealed the expected rapid bone uptake of [131Ba]Ba2+, whereas 131Ba-labeled macropa showed a fast clearance from the blood, thereby showing a significantly (p < 0.001) lower accumulation in the bone. The authors have concluded that barium-131 is a promising SPECT radionuclide and delivers appropriate imaging qualities in small animals. Furthermore, the relative stability of the 131Ba-labeled macropa complex in vivo forms the basis for the development of sufficient new chelators, especially for radium isotopes. Thereby, barium-131 will attain its goal as a diagnostic match to the alpha emitters radium-223 and radium-224.
Indium-111-labeled CD166-targeted peptide as a potential nuclear imaging agent for detecting colorectal cancer stemlike cells in a xenograft mouse model
Siao-Syun Guan1, Cheng-Tien Wu2,3, Tse-Zung Liao1, Tsai-Yueh Luo1, Kun-Liang Lin1, Shing-Hwa Liu4,5,6
1Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan
2Department of Nutrition, China Medical University, Taichung, 40402 Taiwan
3Master Program of Food and Drug Safety, China Medical University, Taichung, 40402 Taiwan
4Institute of Toxicology, College of Medicine, National Taiwan University, No. 1, Jen-Ai Road, Section 1, Taipei, 10051 Taiwan
5Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan
6Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan
https://dx.doi.org/10.1186%2Fs13550-020-0597-3
Summary
Colorectal cancer (CRC) is the third most frequent occurring cancer in men and the second most frequent occurring cancer in women, with nearly 1.65 million new diagnosed cases and about 832,000 deaths in 2015. One possible cause of treatment failure is that the tumor site contains a small population of tumor-initiating cells termed cancer stem cells (CSCs). CSCs are involved in drug resistance, metastasis, and relapse of cancers, which can significantly affect tumor therapy. Hence, to develop specifically therapeutic target probe at CSCs for improvement of survival and quality of life of cancer patients is urgently needed. The CD166 protein has been suggested to be involved in CRC tumorigenesis and to be considered a marker for colorectal CSCs (CRCSCs) detection. In this study, therefore, the authors attend to apply a nuclear imaging agent probe, Glycine18-Cystine-linked CD166-targeted peptides (CD166tp-G18C), to detect the changes of CD166 level in a CRC xenograft mouse model.
Results from the nanoSPECT/CT
For the animal experiment, the authors have used a nanoSPECT/CT, which provided a good enough sensitivity and resolution to make the tumor uptake visible after 2 hours p.i., and see significant differences in the uptake of the applied tracers.
To create a xenograft tumor model, male BALB/c nude mice were subcutaneously inoculating CD166+HCT15 cells (1 × 106 cells) for 2 weeks, and then the 111In-DTPA, 111In-DTPA-G18C, 111In-DTPA-CD166tp-C, and 111In-DTPA-CD166tp-G18C (740 MBq/kg/mouse) were intravenously injected into mice. The imaging of CD166 in mice at 2, 4, 24, and 48 h were detected by the nanoSPECT/CT. For competitive study, the CD166+HCT15-derived xenograft mice were pre-treated with CD166tp-G18C (0, 10, and 50 mg/kg) for 6 h. Every mouse then received 740 MBq/kg 111In-DTPA-CD166tp-G18C via intravenous injection for 24 and 48 h. The competitive CD166 images were observed with the same procedure.
Figure 8. shows the main results from the SPECT/CT acquisitions: The nuclear imaging tracer of 111In-DTPA-CD166tp-G18C for detection of CD166-positive colorectal tumor in vivo. a) The colorectal tumor nuclear imaging analysis in CD166+HCT15 xenograft mice. The 111In-DTPA-CD166tp-G18C and control groups (740 MBq/kg/per mouse) were intravenously injected into mice for 2, 4, 24, and 48 h and detected by a nanoSPECT/CT. Group I, 111In-DTPA; Group II, 111In-DTPA-G18C; Group III, 111In-DTPA-CD166tp-C; Group IV, 111In-DTPA-CD166tp-G18C. b) Quantification of nuclear images in tumor areas of colorectal tumor xenograft mice. The circled positions in images were quantified by a 3D analysis software. Data are presented as mean ± SD (n ≥ 3). *P < 0.05, versus control group. c) The competitive study of 111In-DTPA-CD166tp-G18C in CD166+HCT15 xenograft mice. After tumor xenograft mice were intravenously injected with CD166tp-G18C (0, 10, and 50 mg/kg) for 6 h, 111In-DTPA-CD166tp-G18C (740 MBq/kg/mouse) was intravenously injected into mice for 24 and 48 h and detected by a nanoSPECT/CT. d) Quantification of nuclear images in tumor areas of colorectal tumor xenograft mice. Data are presented as mean ± SD (n ≥ 3). *P < 0.05, versus 0 mg/kg CD166tp-G18C group, #P < 0.05, versus 0 mg/kg CD166tp-G18C group.
- The authors have developed a nuclear imaging agent (111In-DTPA-CD166tp-G18C) using CD166tp-G18C as a probe for CD166-positive CRCs detection in a xenograft mouse model. In this xenograft model, when the tumor size achieved about 150 mm3 which possessed about 1 × 107 CD166-postive cells (cancer cell average diameter: 15 μm), the nanoSPECT/CT detection started to perform.
- These results suggest that CD166-positive CRC exhibited characteristics of CSCs, so it may be a useful drug screening tool for CRC diagnosis. The authors synthesized DTPA-CD166tp-G18C and radiolabeled with Indium-111 for detecting CD166 imaging by using nanoSPECT/CT in CD166-positive CRC xenograft mice. The bio-distribution of 111In-DTPA-CD166tp-G18C confirmed the accumulation of CD166-positive cells in tumors. Therefore, 111In-DTPA-CD166tp-G18C may be a potential nuclear imaging agent for diagnosis of CRCSCs. The CD166 bound peptide-based nuclear imaging may provide physicians to classify cancer cells before treatment and monitor patients with a history of CRC after surgery or drug treatment.

Preclinical Evaluation of a Novel 99mTc-Labeled CB86 for Rheumatoid Arthritis Imaging
Peng Liu1, Tingting Wang1, Rongshui Yang1, Wentao Dong1, Qiang Wang1, Zhide Guo2, Chao Ma1, Weixing Wang1, Huaibo Li1, and Xinhui Su1
1Department of Nuclear Medicine, Zhongshan Hospital Xiamen University, Xiamen 361004, China
2Center for Molecular Imaging and Translational Medicine, Xiamen University, Xiamen 361102, China
https://doi.org/10.1021/acsomega.0c04066
Summary
Early diagnosis and therapy are crucial to control disease progression optimally and achieve a good prognosis in rheumatoid arthritis (RA). Moreover, therapeutic intervention should start as soon as the diagnosis has been established, with the aim of stopping inflammation before irreversible damage is caused, but unfortunately current diagnostic methods are still not very sensitive and specific to RA.
Early hallmark of RA is the increased number of activated macrophages in the synovium with strong increase of their translocator protein (TSPO) level.
In previous studies a 99mtechnetium-labeled TSPO ligand (99mTc-CB256) was used to image a TSPO-rich cancer cell in vitro; however, few 99mTc-CB256 in vivo evaluation has been reported so far probably due to the cytotoxicity of CB256 (75 times more than analogous CB86). Here, a novel TSPO targeting radiopharmaceutical consisting of CB86 and diethylenetriaminepentaacetic acid (DTPA) is described.
Cytotoxicity, binding affinity and specificity of 99mTc-DTPA-CB86 to TSPO were evaluated using RAW264.7 macrophage cells. Biodistribution and 99mTc-SPECT studies were conducted on RA rat models after the injection of 99mTc-DTPA-CB86 with or without co-injection of unlabeled DTPA-CB86.
The probe displayed good stability in vitro and binding specificity to RAW264.7 macrophage cells. In the biodistribution studies, 99mTc-DTPA-CB86 exhibited rapid inflammatory ankle accumulation. At 180 min after administration, 99mTc-DTPA-CB86 uptakes of the left inflammatory ankle were 2.35 ± 0.10 percentage of the injected radioactivity per gram of tissue (% ID/g), significantly higher than those of the normal tissues. 99mTc-SPECT imaging studies revealed that 99mTc-DTPA-CB86 could clearly identify the left inflammatory ankle with good contrast at 30−180 min after injection. Therefore, 99mTc-DTPA-CB86 may be a promising probe for arthritis 99mTc-SPECT imaging.
Results from nanoScan SPECT/CT
The RA rats (n = 4 for each group) were injected with 99mTc-DTPA-CB86 (0.37 MBq, 100 μL) with or without co-injection of unlabeled DTPA-CB86 (300 μg) through the tail vein. At 30, 90, and 180 min after injection, they were anesthetized with 2% isoflurane and placed on the SPECT bed. SPECT acquiring parameters were as follows: a 140 keV energy peak for 99mTc, window width of 20%, a matrix of 256 × 256 and time frame 30 s. Whole-body static images (200 000 counts) were acquired with a matrix of 218 × 218, and a zoom of 2.0. CT data were acquired using an X-ray voltage biased to 50 kVp with a 670 μA current, with #projections 720°. Regions of interest (ROI) were drawn over the left inflammatory ankle and normal muscle, and then the ratios of the left inflammatory ankle to muscle were calculated.
- 99mTc-DTPA-CB86 accumulated in the left inflammatory ankles at 30 min and then showed a gradual increase of uptake. During 90−180 min after injection, the left inflammatory ankles were clearly visible, with good inflammatory to background contrast.
- When co-injected with unlabeled DTPA-CB86 (300 μg), the left inflammatory ankles were barely visible on SPECT images at 30−180 min after injection.
- Regions of interest (ROI) analysis of SPECT showed a high ratio of the left inflammatory ankle to muscle for RA rats injected unblocking dose compared to with 300 μg blocking dose at 30−180 min postinjection (P < 0.05).
- Evaluation of the probe in these RA rats demonstrated that 99mTc-DTPA-CB86 may be a promising agent for TSPO SPECT imaging.

Heterodimeric Radiotracer Targeting PSMA and GRPR for Imaging of Prostate Cancer-Optimization of the Affnity towards PSMA by Linker Modification in Murine Model
Fanny Lundmark1, Ayman Abouzayed1, Bogdan Mitran1,2, Sara S. Rinne1, Zohreh Varasteh1,3, Mats Larhed4 , Vladimir Tolmachev5,6 , Ulrika Rosenström1 and Anna Orlova 1,4,6
1 Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
2 Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet and Stockholm County Council, 171 77 Stockholm, Sweden
3 Department of Nuclear Medicine, Klinikum rechts der Isar der TUM, 80802 Munich, Germany
4 Science for Life Laboratory, Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
5 Department of Immunology, Genetics and Pathology, Uppsala University, 751 83 Uppsala, Sweden
6 Research Centrum for Oncotheranostics, Research School of Chemistry and Applied Biomedical Sciences, Tomsk Polytechnic University, 634050 Tomsk, Russia
doi:10.3390/pharmaceutics12070614
Summary
Prostate-specific membrane antigen (PSMA) and gastrin-releasing peptide receptor (GRPR) are overexpressed in prostate cancer (PCa) cells and are promising targets for molecular imaging methods used for diagnosis. Novel heterodimer - containing PSMA inhibitor and GRPR antagonist - has been demonstrated to bind specifically to both proteins with concomitant low uptake in normal tissues. In the current study, chemical structure of the heterodimer was modified in order to improve affinity towards PCa cells and binding characteristics were analysed. Tumor-bearing mice were injected with 111In-labeled heterodimer (BQ7812). In vivo biodistribution was investigated on harvested organs and also with SPECT/CT imaging. Quantitative analysis together with in vitro tests revealed that modifications in the molecular design resulted in 10-fold improved affnity towards PSMA and high activity uptake in tumors.
Results from nanoScan SPECT/CT
For the SPECT/CT studies, BALB/c nu/nu mice implanted with PC3-pip (isogenic human prostate carcinoma) cells were injected with 830kBq 111In-BQ7812. Groups were also co-injected with non-labeled GRPR antagonist and/or non-labeled PSMA-11 to block GRPR and/or PSMA to prove binding specificity. Imaging of the non-blocked group was performed at 1 and 3h pi and for the GRPR/PSMA-blocked group at 1h pi.
Result revealed that:
- images are in good agreement with the ex vivo analysis: tumor could be visualized already at 1h pi. and the only healthy organs with high activity uptake at this time point were the kidneys (Figure 1.A)
- Co‐injection of non-labeled PSMA-11 and NOTA-PEG4-RM26 resulted in a decreased kidney uptake and a negligible activity uptake in the tumor (Figure 1.B)
- activity cleared from healthy organs and blood with time, leading to an improved imaging contrast at 3h pi. (Figure 1.C)
- ∑ Together with the results from the in vitro and in vivo specificity tests, confirmed the specific binding of [111In]In-BQ7812 to both PSMA and GRPR

Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics
Oskar Vilhelmsson Timmermand1, Jörgen Elgqvist2, Kai A. Beattie3, Anders Örbom1, Erik Larsson4, Sophie E. Eriksson1, Daniel L.J. Thorek5, Bradley J. Beattie6, Thuy A. Tran7,8, David Ulmert1,3,9, Sven-Erik Strand1,4
1Division of Oncology and Pathology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
3Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
4Division of Medical Radiation Physics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
5Department of Radiology, Washington University School of Medicine, Saint Louis, MO, 63108, USA
6Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
7Department of Radiopharmacy, Karolinska University Hospital, Stockholm, Sweden
8Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
9Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at University of California, Los Angeles (UCLA), CA, USA
https://doi.org/10.7150/thno.31179
Summary
Particular metastatic prostate cancers can be treated well with androgen ablating drugs, however resistance is probably developing, and with the increased expression of the Androgen Receptor (AR), the tumor may growths back.
Human Kallikrein 2, which is a downstream molecule of AR pathway, can be a potential target, and the authors have used an antibody (hu11B6) against it. They assessed the efficacy of hu11B6 labeled with a low LET beta-emitter, Lutetium-177 (177Lu) and investigated whether similar tumor killing and AR-enhancement is produced. Moreover, single-photon emission computed tomography (SPECT) imaging of 177Lu is quantitatively accurate and can be used to perform treatment planning. [177Lu]hu11B6 therefore has significant potential as a theranostic agent.
Results from nanoSPECT/CT Plus
For the SPECT/CT studies, the authors have used a nanoSPECT/CT Plus, which is a precise option to follow the biodistribution of 177Luhu11B6, and follow the tumor size in mice with good resolution. Subcutaneous PCa xenografts (LNCaP s.c.) were grown in male mice. Biokinetics at 4-336 h post injection and uptake as a function of the amount of hu11B6 injected at 72 h were studied. Over a 30 to 120-day treatment period the therapeutic efficacy of different activities of [177Lu]hu11B6 were assessed by SPECT/CT imaging besides other options.
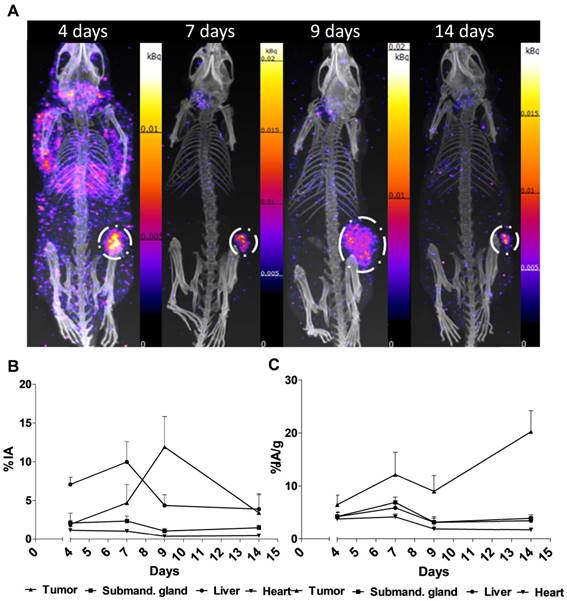
Performing the acquisitions, a multipinhole mouse collimator was used and with energy windows of 20% centered over the 56-, 113-, and 208-keV energy peaks of 177Lu. Acquisition time was about 40 min. With the help of the CT images as an anatomical reference, regions of interest (ROI), where drawn for tumor, submandibular glands, liver and heart.
Figure 2. shows the main results from the SPECT/CT acquisitions: A. Representative maximum intensity projections of SPECT/CT of mice at 4, 7, 9 and 14 days p.i. of 177Lu-hu11B6. B. Biokinetics as percent injected activity per gram of tissue (%IA/g) of therapeutic amounts of 177Lu-hu11B6 (20-30 µg) quantified from SPECT data. Quantified data from SPECT/CT imaging of 177Lu-hu11B6 for tumor, submandibular gland, liver and heart. C. Quantified %IA data from SPECT/CT imaging of 177Lu-hu11B6.
- The results suggest tumor accumulation of [177Lu]hu11B6 peaked at 168 h with a specific uptake of 29 ± 9.1 percent injected activity per gram (%IA/g) and low accumulation in normal organs except in the submandibular gland (15 ± 4.5 %IA/g), attributed to a cross-reaction with mice kallikreins in this organ, was seen. However, SPECT imaging with therapeutic amounts of [177Lu]hu11B6 revealed no peak in tumor accumulation at 7 d, probably due to cellular retention of 177Lu and decreasing tumor volumes.
- This study shows that hu11B6 labeled with the low LET beta-emitting radionuclide 177Lu can deliver therapeutic absorbed doses to prostate cancer xenografts with transient hematological side-effects.
Nanomedicine and Personalized Treatments
Nanomedicine is simply the medical application of nanotechnologies. The idea is the involvement the use of nanoparticles to improve the behaviour of drug substances. The goal is to achieve improvement over conventional chemotherapies. Customized treatments will be required to overcome the issues raised by clinical patient and disease heterogeneity. As one might expect, the same drug will accumulate in tumors at varying concentrations in patients with different cancers. But this also happens in patients with the same kind of cancer. It has to be ensured that drug nanocarriers are really accumulating in the specific tissues to better treat patients. This brings in the necessity of a treatment prediction tool to select the patients most likely to accumulate high amounts of the nanomedicine of interest and hence benefit from nanomedicinal treatment.
Positron Emission Tomography (PET) is such a noninvasive quantitative imaging tool with excellent sensitivity and spatial/temporal resolution required at the whole-body level. Radiolabeling of liposomal nanomedicines with single-photon emission computed tomography (SPECT) radionuclides has been successfully used to study their biodistribution in preclinical and clinical studies, but SPECT imaging suffers from lower sensitivity and temporal/spatial resolution than PET. However, an ideal PET radiolabeling method viable for both preclinical and clinical imaging wasn’t explored before. Rafael T. M. de Rosales, Alberto Gabizon and colleagues at King’s College London and the Shaare Zedek Medical Center sought to address this challenge.
 Edmonds, S. et al. Exploiting the Metal-Chelating Properties of the Drug Cargo for In Vivo Positron Emission Tomography Imaging of Liposomal Nanomedicines. ACS Nano (2016). doi:10.1021/acsnano.6b05935
Edmonds, S. et al. Exploiting the Metal-Chelating Properties of the Drug Cargo for In Vivo Positron Emission Tomography Imaging of Liposomal Nanomedicines. ACS Nano (2016). doi:10.1021/acsnano.6b05935
The following Mediso systems were used to conduct the animal imaging studies: nanoScan PET/CT and NanoSPECT/CT Silver upgrade. Both systems are equipped with the MultiCell animal handling and monitoring system , thus enabling a combined PET-CT/SPECT-CT imaging strategy. Interestingly both PET and SPECT were performed in the same animals (by moving the same bed from scanner from scanner, while the animals were anesthetized in fixed position) that allowed to image the tumour cells with SPECT and the nanomedicine with PET.
Liposomal Drug PET Radiolabeling Method Development
The researchers introduced a simple and efficient PET radiolabeling method exploiting the metal-chelating properties of certain drugs (e.g., bisphosphonates such as alendronate and anthracyclines such as doxorubicin) and widely used ionophores radiolabeled with long half-life metallic PET isotopes, such as 89Zr, 52Mn and 64Cu. The labels — and thus the liposomal drugs — could then be tracked using positron emission tomography (PET) to see where they go within the body. The article discusses in details the feasibility and effectiveness of their method, as well as its advantages and limitations, and show its utility for detecting and quantifying the biodistribution of a liposomal nanomedicine containing an aminobisphosphonate in vivo.
 In a model of metastatic breast cancer, the researchers demonstrated that their technique allows quantification of the biodistribution of a radiolabeled stealth liposomal nanomedicine. Alendronate (ALD), an aminobisphosphonate, was selected as the radionuclide-binding drug of choice to develop this method for two reasons: (i) known ability to act as metal chelator to form inert coordination complexes with zirconium, copper, and manganese; and (ii) demonstrated anticancer activity and γ−δ T-cell immunotherapy sensitizing properties. The used liposomal formulation is referred to as PLA in the article.
In a model of metastatic breast cancer, the researchers demonstrated that their technique allows quantification of the biodistribution of a radiolabeled stealth liposomal nanomedicine. Alendronate (ALD), an aminobisphosphonate, was selected as the radionuclide-binding drug of choice to develop this method for two reasons: (i) known ability to act as metal chelator to form inert coordination complexes with zirconium, copper, and manganese; and (ii) demonstrated anticancer activity and γ−δ T-cell immunotherapy sensitizing properties. The used liposomal formulation is referred to as PLA in the article.
Monitoring Liposomal Nanomedicine Distribution
The biodistribution of the radiolabeled liposomes was monitored using PET imaging with 89Zr-PLA in a metastatic mammary carcinoma mouse model established in immunocompromised NSG mice. This cancer model is also traceable by SPECT imaging/fluorescence due to a dual-modality reporter gene, the human sodium iodide symporter (hNIS-tagRFP), that allows sensitive detection of viable cancer tissues (primary tumor and metastases) using SPECT imaging with 99mTc-pertechnetate and fluorescence during dissection and histological studies. The imaging protocol was as follows: first, mice were injected with 89Zr-PLA (4.6 ± 0.4 MBq) at t = 0 followed by nanoScan PET-CT imaging (liposome biodistribution). The same mice were then injected with 99mTc-pertechnetate (30 MBq) and imaged by SPECT-CT. The SPECT injection was repeated at t = 24 h, 72 h, and 168 h. It was confirmed by separate phantom studies that the presence of 99mTc was not affecting the quality/quantification of the PET study. CT images revealed a significant increase in tumor volume during the imaging study. Using the tumor volumes from SPECT and CT, the researchers calculated the percentage of necrotic tumor tissue over time, by subtracting the hNIS-positive volume (SPECT) to the total tumor volume (CT). A PET-CT study was also performed using 64Cu-PLA in an ovarian cancer model (SKOV-3/SCID-Beige) over 48 h to test the versatility and capability of the radiolabeling method.
The common MultiCell animal handling and monitoring system (developed by Mediso) on both imaging systems gave the possibility to easily co-register the PET-CT/SPECT-CT and PET/SPECT studies as the animals were moved in co-registered position between the systems.
MIP video (3D, rotating along z-axis) showing co-registration of PET (red signal, 89Zr-PLA) and SPECT (green signal, 99mTcO4-, hNIS positive viable tumour tissue) of representative tumor from the mutimodal PET/SPECT study in the 3E.Δ.NT/NSG model. Both signals/radiotracers accumulate predominantly at the rim of the tumour and areas of low colocalization as well as high co-localization (yellow) are evident.
Imaging with PET in mouse models of breast and ovarian cancer showed the drugs accumulated in tumors and metastatic tissues in varying concentrations and at levels well above those in normal tissues, the researchers report. In one mouse strain, the nanomedicines unexpectedly showed up in uteruses, a result that wouldn’t have been detected without conducting the imaging study, according to the researchers.
Discussion
The results establish that preformed liposomal nanomedicines, including some currently in clinical use, can be efficiently labeled with PET radiometals and tracked in vivo by exploiting the metal affinity and high concentration of the encapsulated drugs. Importantly, the technique allows radiolabeling of preformed liposomal nanomedicines, without modification of their components and without affecting their physicochemical properties.
The versatility, efficiency, simplicity, and GMP compatibility of this method may enable submicrodosing imaging studies of liposomal nanomedicines containing chelating drugs in humans and may have clinical impact by facilitating the introduction of image-guided therapeutic strategies in current and future nanomedicine clinical studies. The ultimate goal is to use non-invasive imaging data to predict how much drug will be delivered to cancer tissues in specific patients, and whether the nanomedicine is reaching all the patient’s tumors in therapeutic concentrations.
Many thanks for Rafael T. M. de Rosales, the last author of the original article.
There is a nanoScan SPECT/CT in Boston - installed after Thanksgiving - stay tuned for more information.