Innovation Delivered
Heterodimeric Radiotracer Targeting PSMA and GRPR for Imaging of Prostate Cancer-Optimization of the Affnity towards PSMA by Linker Modification in Murine Model
Fanny Lundmark1, Ayman Abouzayed1, Bogdan Mitran1,2, Sara S. Rinne1, Zohreh Varasteh1,3, Mats Larhed4 , Vladimir Tolmachev5,6 , Ulrika Rosenström1 and Anna Orlova 1,4,6
1 Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
2 Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet and Stockholm County Council, 171 77 Stockholm, Sweden
3 Department of Nuclear Medicine, Klinikum rechts der Isar der TUM, 80802 Munich, Germany
4 Science for Life Laboratory, Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
5 Department of Immunology, Genetics and Pathology, Uppsala University, 751 83 Uppsala, Sweden
6 Research Centrum for Oncotheranostics, Research School of Chemistry and Applied Biomedical Sciences, Tomsk Polytechnic University, 634050 Tomsk, Russia
doi:10.3390/pharmaceutics12070614
Summary
Prostate-specific membrane antigen (PSMA) and gastrin-releasing peptide receptor (GRPR) are overexpressed in prostate cancer (PCa) cells and are promising targets for molecular imaging methods used for diagnosis. Novel heterodimer - containing PSMA inhibitor and GRPR antagonist - has been demonstrated to bind specifically to both proteins with concomitant low uptake in normal tissues. In the current study, chemical structure of the heterodimer was modified in order to improve affinity towards PCa cells and binding characteristics were analysed. Tumor-bearing mice were injected with 111In-labeled heterodimer (BQ7812). In vivo biodistribution was investigated on harvested organs and also with SPECT/CT imaging. Quantitative analysis together with in vitro tests revealed that modifications in the molecular design resulted in 10-fold improved affnity towards PSMA and high activity uptake in tumors.
Results from nanoScan SPECT/CT
For the SPECT/CT studies, BALB/c nu/nu mice implanted with PC3-pip (isogenic human prostate carcinoma) cells were injected with 830kBq 111In-BQ7812. Groups were also co-injected with non-labeled GRPR antagonist and/or non-labeled PSMA-11 to block GRPR and/or PSMA to prove binding specificity. Imaging of the non-blocked group was performed at 1 and 3h pi and for the GRPR/PSMA-blocked group at 1h pi.
Result revealed that:
- images are in good agreement with the ex vivo analysis: tumor could be visualized already at 1h pi. and the only healthy organs with high activity uptake at this time point were the kidneys (Figure 1.A)
- Co‐injection of non-labeled PSMA-11 and NOTA-PEG4-RM26 resulted in a decreased kidney uptake and a negligible activity uptake in the tumor (Figure 1.B)
- activity cleared from healthy organs and blood with time, leading to an improved imaging contrast at 3h pi. (Figure 1.C)
- ∑ Together with the results from the in vitro and in vivo specificity tests, confirmed the specific binding of [111In]In-BQ7812 to both PSMA and GRPR

Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics
Oskar Vilhelmsson Timmermand1, Jörgen Elgqvist2, Kai A. Beattie3, Anders Örbom1, Erik Larsson4, Sophie E. Eriksson1, Daniel L.J. Thorek5, Bradley J. Beattie6, Thuy A. Tran7,8, David Ulmert1,3,9, Sven-Erik Strand1,4
1Division of Oncology and Pathology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
3Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
4Division of Medical Radiation Physics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
5Department of Radiology, Washington University School of Medicine, Saint Louis, MO, 63108, USA
6Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
7Department of Radiopharmacy, Karolinska University Hospital, Stockholm, Sweden
8Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
9Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at University of California, Los Angeles (UCLA), CA, USA
https://doi.org/10.7150/thno.31179
Summary
Particular metastatic prostate cancers can be treated well with androgen ablating drugs, however resistance is probably developing, and with the increased expression of the Androgen Receptor (AR), the tumor may growths back.
Human Kallikrein 2, which is a downstream molecule of AR pathway, can be a potential target, and the authors have used an antibody (hu11B6) against it. They assessed the efficacy of hu11B6 labeled with a low LET beta-emitter, Lutetium-177 (177Lu) and investigated whether similar tumor killing and AR-enhancement is produced. Moreover, single-photon emission computed tomography (SPECT) imaging of 177Lu is quantitatively accurate and can be used to perform treatment planning. [177Lu]hu11B6 therefore has significant potential as a theranostic agent.
Results from nanoSPECT/CT Plus
For the SPECT/CT studies, the authors have used a nanoSPECT/CT Plus, which is a precise option to follow the biodistribution of 177Luhu11B6, and follow the tumor size in mice with good resolution. Subcutaneous PCa xenografts (LNCaP s.c.) were grown in male mice. Biokinetics at 4-336 h post injection and uptake as a function of the amount of hu11B6 injected at 72 h were studied. Over a 30 to 120-day treatment period the therapeutic efficacy of different activities of [177Lu]hu11B6 were assessed by SPECT/CT imaging besides other options.
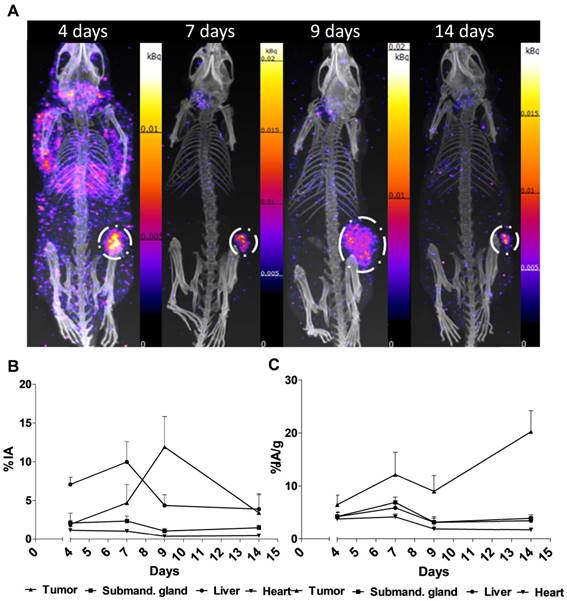
Performing the acquisitions, a multipinhole mouse collimator was used and with energy windows of 20% centered over the 56-, 113-, and 208-keV energy peaks of 177Lu. Acquisition time was about 40 min. With the help of the CT images as an anatomical reference, regions of interest (ROI), where drawn for tumor, submandibular glands, liver and heart.
Figure 2. shows the main results from the SPECT/CT acquisitions: A. Representative maximum intensity projections of SPECT/CT of mice at 4, 7, 9 and 14 days p.i. of 177Lu-hu11B6. B. Biokinetics as percent injected activity per gram of tissue (%IA/g) of therapeutic amounts of 177Lu-hu11B6 (20-30 µg) quantified from SPECT data. Quantified data from SPECT/CT imaging of 177Lu-hu11B6 for tumor, submandibular gland, liver and heart. C. Quantified %IA data from SPECT/CT imaging of 177Lu-hu11B6.
- The results suggest tumor accumulation of [177Lu]hu11B6 peaked at 168 h with a specific uptake of 29 ± 9.1 percent injected activity per gram (%IA/g) and low accumulation in normal organs except in the submandibular gland (15 ± 4.5 %IA/g), attributed to a cross-reaction with mice kallikreins in this organ, was seen. However, SPECT imaging with therapeutic amounts of [177Lu]hu11B6 revealed no peak in tumor accumulation at 7 d, probably due to cellular retention of 177Lu and decreasing tumor volumes.
- This study shows that hu11B6 labeled with the low LET beta-emitting radionuclide 177Lu can deliver therapeutic absorbed doses to prostate cancer xenografts with transient hematological side-effects.