Innovation Delivered
Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging
Falco Reissig1, David Bauer1,2, Martin Ullrich1, Martin Kreller1, Jens Pietzsch1,2, Constantin Mamat1,2, Klaus Kopka1,2, Hans-Jürgen Pietzsch1, Martin Walther1
1Helmholtz-Zentrum Dresden-Rossendorf, Institut für Radiopharmazeutische Krebsforschung, Bautzner Landstraße 400, D-01328 Dresden, Germany
2Fakultät Chemie und Lebensmittelchemie, Technische Universität Dresden, D-01062 Dresden, Germany
https://doi.org/10.3390/ph13100272
Summary
Barium-131 is a single photon emission computed tomography (SPECT)-compatible radionuclide for nuclear medicine and a promising diagnostic match for Radium-223/-224. In the early 1970s, Barium-131 has been thoroughly investigated as a potential bone targeting radiotracer, but no substantial benefits have been mentioned, comparing it to other already applicable radiotracers like [18F]F− (t½ = 110 min) and 99mTc-labeled (t½ = 6.0 h) bisphosphonates. However, as part of current approaches to the therapy of bone cancer and bone metastases, this radionuclide has its significance in modern times. Barium-131 possesses the suitable half-life of 11.5 d, thereby making it highly beneficial for potential diagnostic use in nuclear medicine. Due to the similar chemistry and pharmacological properties of the elements Barium and Radium, Barium-131 is particularly feasible as a diagnostic match to the therapeutic α-emitters Radium-223 and Radium-224. In the work presented here, the authors aimed to establish a simple but sufficient procedure for the production and purification of n.c.a. Barium-131 using the TR-FLEX cyclotron (ACSI), starting from a cheap Cesium Chloride target with natural monoisotopically occurring Cesium followed by 27.5 MeV proton bombardment. Moreover, the in-house produced Barium-131 was used for first labeling studies with the chelator macropa, for initial in vivo-related phantom studies and, last but not least, small animal imaging trials with [131Ba]Ba(NO3)2 and 131Ba-labeled macropa in healthy mice.
Results from the nanoScan SPECT/CT
For the small animal imaging, the authors have used a nanoScan SPECT/CT, to follow the biodistribution with the different Barium-131 tracers.
SPECT/CT imaging in mice was performed at 1 h and 24 h after i.v. injection of [131Ba]Ba(NO3)2 (6.2 MBq in 0.2 mL of 0.01 M HNO3, pH 6, Am = 420 GBq/µmol, n.c.a.), or 131Ba-labeled macropa (6.7 MBq in 0.2 mL of 0.1 M ammonium acetate, pH 6, Am = 83 MBq/µmol) with a frame time of 60 s (total scan time: 1.5 h), respectively. The acquisition was performed using a standard aperture for mouse imaging (APT62) consisting of four M3 multi-pinhole collimators providing a 30 × 30 mm transaxial field of view (FOV). Projection data were reconstructed using the Tera-Tomo™ 3D high dynamic range algorithm (resolution: 128; iterations: 48; subset size: 4), applying corrections for decay, scatter, and attenuation.
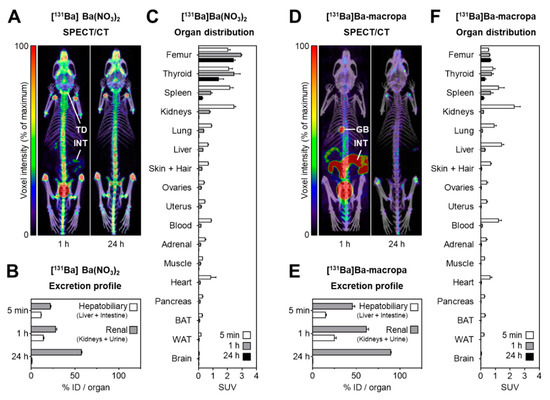
Figure 8. shows the distribution of [131Ba]Ba(NO3)2 and [131Ba]Ba-macropa in mice. (A) SPECT/CT fusion images of [131Ba]Ba(NO3)2 in a mouse 1 h and 24 h after injection; (B,C) excretion profile and organ distribution of [131Ba]Ba(NO3)2 in mice 5 min, 1 h, and 24 h after injection (n = 4); (D) SPECT/CT fusion images of [131Ba]Ba-macropa in a mouse 1 h and 24 h after injection; (E,F) excretion profile and organ distribution of [131Ba]Ba-macropa in mice; 5 min, 1 h, and 24 h after injection (n = 4); (BAT) brown adipose tissue; (GB) gall bladder *; (ID) initial dose; (INT) intestine; (TD) thyroid/parathyroid *; (WAT) white adipose tissue (* activity in these organs was not measured separately).
- The author have shown for the first time the in vivo biodistribution behavior of 131Ba-labeled macropa in comparison with free [131Ba]Ba2+ by means of small animal SPECT/CT.
- Biodistribution studies revealed the expected rapid bone uptake of [131Ba]Ba2+, whereas 131Ba-labeled macropa showed a fast clearance from the blood, thereby showing a significantly (p < 0.001) lower accumulation in the bone. The authors have concluded that barium-131 is a promising SPECT radionuclide and delivers appropriate imaging qualities in small animals. Furthermore, the relative stability of the 131Ba-labeled macropa complex in vivo forms the basis for the development of sufficient new chelators, especially for radium isotopes. Thereby, barium-131 will attain its goal as a diagnostic match to the alpha emitters radium-223 and radium-224.
Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics
Oskar Vilhelmsson Timmermand1, Jörgen Elgqvist2, Kai A. Beattie3, Anders Örbom1, Erik Larsson4, Sophie E. Eriksson1, Daniel L.J. Thorek5, Bradley J. Beattie6, Thuy A. Tran7,8, David Ulmert1,3,9, Sven-Erik Strand1,4
1Division of Oncology and Pathology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
3Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
4Division of Medical Radiation Physics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
5Department of Radiology, Washington University School of Medicine, Saint Louis, MO, 63108, USA
6Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
7Department of Radiopharmacy, Karolinska University Hospital, Stockholm, Sweden
8Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
9Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at University of California, Los Angeles (UCLA), CA, USA
https://doi.org/10.7150/thno.31179
Summary
Particular metastatic prostate cancers can be treated well with androgen ablating drugs, however resistance is probably developing, and with the increased expression of the Androgen Receptor (AR), the tumor may growths back.
Human Kallikrein 2, which is a downstream molecule of AR pathway, can be a potential target, and the authors have used an antibody (hu11B6) against it. They assessed the efficacy of hu11B6 labeled with a low LET beta-emitter, Lutetium-177 (177Lu) and investigated whether similar tumor killing and AR-enhancement is produced. Moreover, single-photon emission computed tomography (SPECT) imaging of 177Lu is quantitatively accurate and can be used to perform treatment planning. [177Lu]hu11B6 therefore has significant potential as a theranostic agent.
Results from nanoSPECT/CT Plus
For the SPECT/CT studies, the authors have used a nanoSPECT/CT Plus, which is a precise option to follow the biodistribution of 177Luhu11B6, and follow the tumor size in mice with good resolution. Subcutaneous PCa xenografts (LNCaP s.c.) were grown in male mice. Biokinetics at 4-336 h post injection and uptake as a function of the amount of hu11B6 injected at 72 h were studied. Over a 30 to 120-day treatment period the therapeutic efficacy of different activities of [177Lu]hu11B6 were assessed by SPECT/CT imaging besides other options.
Performing the acquisitions, a multipinhole mouse collimator was used and with energy windows of 20% centered over the 56-, 113-, and 208-keV energy peaks of 177Lu. Acquisition time was about 40 min. With the help of the CT images as an anatomical reference, regions of interest (ROI), where drawn for tumor, submandibular glands, liver and heart.
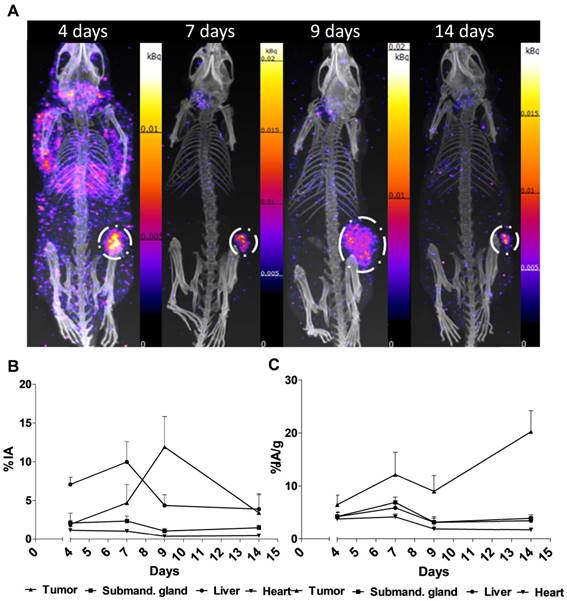
Figure 2. shows the main results from the SPECT/CT acquisitions: A. Representative maximum intensity projections of SPECT/CT of mice at 4, 7, 9 and 14 days p.i. of 177Lu-hu11B6. B. Biokinetics as percent injected activity per gram of tissue (%IA/g) of therapeutic amounts of 177Lu-hu11B6 (20-30 µg) quantified from SPECT data. Quantified data from SPECT/CT imaging of 177Lu-hu11B6 for tumor, submandibular gland, liver and heart. C. Quantified %IA data from SPECT/CT imaging of 177Lu-hu11B6.
- The results suggest tumor accumulation of [177Lu]hu11B6 peaked at 168 h with a specific uptake of 29 ± 9.1 percent injected activity per gram (%IA/g) and low accumulation in normal organs except in the submandibular gland (15 ± 4.5 %IA/g), attributed to a cross-reaction with mice kallikreins in this organ, was seen. However, SPECT imaging with therapeutic amounts of [177Lu]hu11B6 revealed no peak in tumor accumulation at 7 d, probably due to cellular retention of 177Lu and decreasing tumor volumes.
- This study shows that hu11B6 labeled with the low LET beta-emitting radionuclide 177Lu can deliver therapeutic absorbed doses to prostate cancer xenografts with transient hematological side-effects.