Innovation Delivered
Spatiotemporal in vivo tracking of polyclonal human regulatory T cells (Tregs) reveals a role for innate immune cells in Treg transplant recruitment
Jacinta Jacob1, Suchita Nadkarni2, Alessia Volpe3,6, Qi Peng1, Sim L. Tung1, Rosalind F. Hannen2,
Yasmin R. Mohseni1,3, Cristiano Scotta1, Federica M. Marelli-Berg4, Robert I. Lechler1, Lesley A. Smyth1,5,7, Gilbert O. Fruhwirth3,7, and Giovanna Lombardi1,7
1MRC Centre for Transplantation, Peter Gorer Department of Immunobiology, School of Immunology and Microbial Science, King’s College London, Guy’s Hospital, London SE1 9RT, UK;
2Centre for Cell Biology & Cutaneous Research, The Blizard Institute, Bart’s and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK;
3Imaging Therapies and Cancer Group, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK;
4William Harvey Research Institute, Bart’s and The London School of Medicine, Queen Mary University of London, London EC1M 6BQ, UK;
5School of Health, Sport and Bioscience, Stratford Campus, University of East London, London E16 2RD, UK
6Present address: Molecular Imaging Group, Department of Radiology, Memorial Sloan Kettering Cancer Center, 417 E 68th St., New York, NY 10065, USA
7Senior author
https://doi.org/10.1016/j.omtm.2020.12.003
Summary
Regulatory T cells (Tregs) have gained important role in mechanisms of transplantation tolerance and graft survival due to their putative capability to control immune responses. They can prolong the survival of allografts when they are administered after their purification from blood and further manipulated in vitro.
Despite an increase in the number of clinical trials using Treg therapy, important questions remain unclear such as their in vivo biodistribution over couple of weeks.
The aim of the present study was to investigate their long-term (for up to 40 days) biodistribution in vivo by SPECT imaging in mice bearing human skin transplants. As long-term tracking is challenging if radioactive dose has to be minimized, transplanted mice received genetically modified human Treg cells: they were lentivirally transduced with the human sodium iodide symporter (hNIS) and for the imaging, its alternative substrate, pertechnetate (99mTcO4¯) was injected iv. on the days of the scan. In one group of mice Gr-1+ cells (including neutrophils and certain monocytes) were depleted using an antibody raised against Gr-1 to achieve a high level of immunocompromise.
Results show that 99mTcO4¯ uptake was elevated much earlier in the presence of Gr-1+ cells, suggesting their active, accelerating role in influencing Treg recruitment to the graft.
Results from nanoScan SPECT/CT
Skin grafts were transplanted onto 10-12-week-old recipient BRG or NSG mice. 5–6 week later, 5x106 peripheral blood mononuclear cells (PBMCs) were then administered iv. with or without 5x106 Tregs. Some BRG mice received 100mg anti-mouse Gr-1 ip. every two days.
For SPECT/CT imaging 20 MBq 99mTcO4¯ was administered iv. and SPECT scans were acquired 40 min later with nanoScan SPECT/CT. Data were reconstructed using Tera-Tomo with corrections for attenuation, detector dead time, and radioisotope decay in place as needed. CT images were used to draw ROIs and provide the volumes required for standard uptake value calculations. The total activity in the whole animal (excluding the tail) at the time of tracer administration was defined as the injected dose (ID).
- Serial SPECT/CT imaging of mice received Cr-1 antibody revealed that radiotracer uptake in the human skin grafts did not differ from control animals in the first 2 weeks, but at late time points (ranging 30–40 days after administration) Treg presence was significantly elevated.
- In case of the presence of Gr-1+ cells, Tregs were detectable at the skin grafts as early as 3 days after administration. Their signals peaked at around 8 days and remained detectable in the transplants up to 40 days after administration. Early trafficking of Tregs to the skin in the presence of Gr-1+ cells suggested an active, accelerating role of these cells in influencing Treg recruitment to the graft.

Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging
Falco Reissig1, David Bauer1,2, Martin Ullrich1, Martin Kreller1, Jens Pietzsch1,2, Constantin Mamat1,2, Klaus Kopka1,2, Hans-Jürgen Pietzsch1, Martin Walther1
1Helmholtz-Zentrum Dresden-Rossendorf, Institut für Radiopharmazeutische Krebsforschung, Bautzner Landstraße 400, D-01328 Dresden, Germany
2Fakultät Chemie und Lebensmittelchemie, Technische Universität Dresden, D-01062 Dresden, Germany
https://doi.org/10.3390/ph13100272
Summary
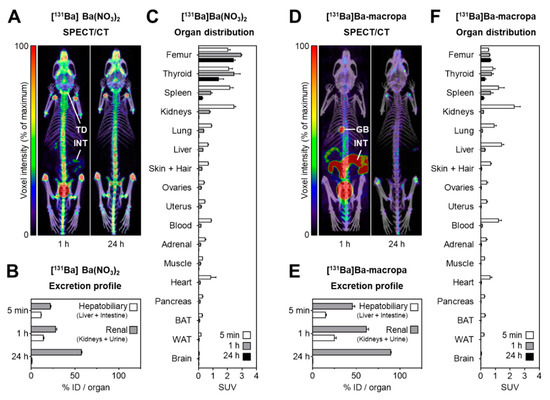
Barium-131 is a single photon emission computed tomography (SPECT)-compatible radionuclide for nuclear medicine and a promising diagnostic match for Radium-223/-224. In the early 1970s, Barium-131 has been thoroughly investigated as a potential bone targeting radiotracer, but no substantial benefits have been mentioned, comparing it to other already applicable radiotracers like [18F]F− (t½ = 110 min) and 99mTc-labeled (t½ = 6.0 h) bisphosphonates. However, as part of current approaches to the therapy of bone cancer and bone metastases, this radionuclide has its significance in modern times. Barium-131 possesses the suitable half-life of 11.5 d, thereby making it highly beneficial for potential diagnostic use in nuclear medicine. Due to the similar chemistry and pharmacological properties of the elements Barium and Radium, Barium-131 is particularly feasible as a diagnostic match to the therapeutic α-emitters Radium-223 and Radium-224. In the work presented here, the authors aimed to establish a simple but sufficient procedure for the production and purification of n.c.a. Barium-131 using the TR-FLEX cyclotron (ACSI), starting from a cheap Cesium Chloride target with natural monoisotopically occurring Cesium followed by 27.5 MeV proton bombardment. Moreover, the in-house produced Barium-131 was used for first labeling studies with the chelator macropa, for initial in vivo-related phantom studies and, last but not least, small animal imaging trials with [131Ba]Ba(NO3)2 and 131Ba-labeled macropa in healthy mice.
Results from the nanoScan SPECT/CT
For the small animal imaging, the authors have used a nanoScan SPECT/CT, to follow the biodistribution with the different Barium-131 tracers.
SPECT/CT imaging in mice was performed at 1 h and 24 h after i.v. injection of [131Ba]Ba(NO3)2 (6.2 MBq in 0.2 mL of 0.01 M HNO3, pH 6, Am = 420 GBq/µmol, n.c.a.), or 131Ba-labeled macropa (6.7 MBq in 0.2 mL of 0.1 M ammonium acetate, pH 6, Am = 83 MBq/µmol) with a frame time of 60 s (total scan time: 1.5 h), respectively. The acquisition was performed using a standard aperture for mouse imaging (APT62) consisting of four M3 multi-pinhole collimators providing a 30 × 30 mm transaxial field of view (FOV). Projection data were reconstructed using the Tera-Tomo™ 3D high dynamic range algorithm (resolution: 128; iterations: 48; subset size: 4), applying corrections for decay, scatter, and attenuation.
Figure 8. shows the distribution of [131Ba]Ba(NO3)2 and [131Ba]Ba-macropa in mice. (A) SPECT/CT fusion images of [131Ba]Ba(NO3)2 in a mouse 1 h and 24 h after injection; (B,C) excretion profile and organ distribution of [131Ba]Ba(NO3)2 in mice 5 min, 1 h, and 24 h after injection (n = 4); (D) SPECT/CT fusion images of [131Ba]Ba-macropa in a mouse 1 h and 24 h after injection; (E,F) excretion profile and organ distribution of [131Ba]Ba-macropa in mice; 5 min, 1 h, and 24 h after injection (n = 4); (BAT) brown adipose tissue; (GB) gall bladder *; (ID) initial dose; (INT) intestine; (TD) thyroid/parathyroid *; (WAT) white adipose tissue (* activity in these organs was not measured separately).
- The author have shown for the first time the in vivo biodistribution behavior of 131Ba-labeled macropa in comparison with free [131Ba]Ba2+ by means of small animal SPECT/CT.
- Biodistribution studies revealed the expected rapid bone uptake of [131Ba]Ba2+, whereas 131Ba-labeled macropa showed a fast clearance from the blood, thereby showing a significantly (p < 0.001) lower accumulation in the bone. The authors have concluded that barium-131 is a promising SPECT radionuclide and delivers appropriate imaging qualities in small animals. Furthermore, the relative stability of the 131Ba-labeled macropa complex in vivo forms the basis for the development of sufficient new chelators, especially for radium isotopes. Thereby, barium-131 will attain its goal as a diagnostic match to the alpha emitters radium-223 and radium-224.
Blood-triggered generation of platinum nanoparticle functions as an anti-cancer agent
Xin Zeng, Jie Sun, Suping Li, Jiyun Shi, Han Gao, Wei Sun Leong, Yiqi Wu, Minghui Li, Chengxin Liu, Ping Li, Jing Kong, Yi-Zhou Wu, Guangjun Nie, Yuming Fu, Gen Zhang
![]() https://doi.org/10.1038/s41467-019-14131-z
https://doi.org/10.1038/s41467-019-14131-z
Summary
Despite the large amount of research on the effects of metal nanoparticles (NPs) in nature and medicine, there has been very limited application in the clinic due to their potential toxicity, cost, and ethical hurdles of research in humans.
In this Nature Communications article, the authors have discovered that platinum (Pt) nanoparticles (NPs) are generated in vivo in human blood when a patient is treated with cisplatin, a powerful anti-cancer agent. They have shown that the self-assembled Pt NPs form rapidly, accumulate in tumors, and remain in the body for an extended period. Furthermore, the Pt NPs by themselves act as anti-cancer agent, but the tumor inhibitory activity is greatly increased when the nanoparticles are loaded with a chemotherapeutic drug, daunorubicin (DNR). The Daunorubicin loaded nanoparticles appeared to be effective even in daunorubicin-resistant models.
The authors proposed that in vivo-generated metal NPs represent a biocompatible drug delivery platform for chemotherapy resistant tumor treatment.
Results from nanoScan SPECT/CT
Authors have used nanoScan SPECT/CT to create high resolution images to track the tumor targeting dynamics of the nanoparticles in vivo.
Human-derived Pt NPs were labeled with 125-I and 500 μCi 125I-Pt NPs was directly injected into DNR-resistant K562-xenografted nude mice. The images were acquired for 30 min at 1, 4, 24 and 48 h time point.
The radioactive signal accumulated in the tumor regions, peaking at 24 h and remaining apparent at 48 h P.I. (Fig. 4f), indicating that the Pt NPs were efficiently taken up by the tumors.

Figure 4. f NanoScan SPECT/CT imaging of 125I-Pt NPs in DNR-resistant K562 cell-xenografted nude mice (n = 5) at 1, 4, 24 and 48 h after intravenous injection of the NPs. The arrows and dotted circles indicate the tumors. MIP: Maximum Intensity Projection.
Nuclear imaging-guided PD-L1blockade therapy increases effectiveness of cancer immunotherapy
Hannan Gao1, Yue Wu 1, Jiyun Shi2, Xin Zhang1, Tianyu Liu1, Biao Hu1, Bing Jia1, Yakun Wan3, Zhaofei Liu1, Fan Wang1,2,4
1Medical Isotopes Research Center and Department of Radiation Medicine, State Key Laboratory of Natural and Biomimetic Drugs, School of Basic Medical Sciences, Peking University, Beijing, China
2Key Laboratory of Protein and Peptide Pharmaceuticals, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
3Shanghai Novamab Biopharmaceuticals Co., Ltd, Shanghai, China
4Bioland Laboratory (Guangzhou Regenerative Medicine and Health Guangdong Laboratory), Guangzhou, China
Summary
The interaction between programmed death receptor-1 (PD-1) and its ligand (PD-L1) inhibits the function of effector T cells and the priming of naive T cells, leading to impaired antitumor immunity. Therefore the blockade of PD-1/PD-L1 signaling pathway has been a breakthrough in cancer therapy, but the response rate in solid tumors is only 20-30%. In this study, a new radiolabeled nanobody-based imaging probe 99mTc-MY1523 targeting PD-L1 was developed for the enhanced therapeutic efficacy of PD-L1 blockade immunotherapy.
Results show that the new probe has high binding affinity and specificity to PD-L1. SPECT/CT imaging revealed fast blood clearance, renal-route excretion and satisfactory tumor uptake.
As the timing for PD-L1 blockade therapy is crucial due to dynamic and heterogeneous expression of PD-L1 in tumors, it was essential to prove that SPECT/CT imaging is able to detect changes in the PD-L1 expression. Therefore PD-L1 expression was increased by interferon-γ (IFN-γ) treatment of tumor bearing mice and PD-L1 expression was determined by SPECT/CT imaging and verified by the flow cytometry. It proves that SPECT/CT imaging of 99mTc-MY1523 can be used to monitor PD-L1 expression in tumors in a real time, dynamic and quantitative manner.
The PD-L1 blockade therapy initiated during the therapeutic time window determined by 99mTc-MY1523 SPECT/CT imaging significantly enhanced the therapeutic efficacy: the tumor growth was dramatically suppressed, and the survival time of mice was evidently prolonged.
Results from nanoScan SPECT/CT
Three types of tumor cells (4T1, A20 or MC-38) were inoculated subcutaneously into the right flank of BALB/c or C57/BL6 mice, respectively. Four days later they were injected i.t. with PBS or IFN-γ for 5 days, and then were subjected to SPECT/CT imaging.
Mice were injected intraveneously with 18 MBq 99mTc-MY1523 and imaged at 2 hours p.i. (n=4) using the nanoScan SPECT/CT system with the following parameters: pinhole SPECT (peak: 140 keV, 20% width; frame time: 25 s), helical CT (50 kVp, 0.67 mA, rotation 210°, exposure time: 300 ms). SPECT and CT images were merged using the Nucline software V.2.0 (Mediso Ltd.). The regions of interest were drawn for the determination of tumor sizes (mm3) and radioactivity (Bq), then the tumor uptake was calculated as percentage injected dose per volume (%ID/cc).
- Results show increased tumor uptake of 99mTc-MY1523 compared to the corresponding control group in all animal models:

When imaging results showed the upregulated PD-L1 expression in tumors after IFN-γ intervention on day 8 and 12 after tumor cell inoculation, the mice were subjected to PD-L1 blockade therapy: they were ip. injected with 200μg αPD-L1 antibody twice with 4 days interval, while using PBS, IFN-γ and αPD-L1 antibody without IFN-γ intervention as controls. Tumor sizes were measured twice a week and calculated as volumes (mm3)=length×width×height/2.
- As shown on the figure below, although IFN-γ intervention expedited the tumor growth, the imaging-guided therapy dramatically improved the therapeutic efficacy. The tumor growth was significantly suppressed, and three of five tumors completely disappeared. Compared to control groups, the survival time of mice in the treated group was also remarkably prolonged.

Indium-111-labeled CD166-targeted peptide as a potential nuclear imaging agent for detecting colorectal cancer stemlike cells in a xenograft mouse model
Siao-Syun Guan1, Cheng-Tien Wu2,3, Tse-Zung Liao1, Tsai-Yueh Luo1, Kun-Liang Lin1, Shing-Hwa Liu4,5,6
1Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan
2Department of Nutrition, China Medical University, Taichung, 40402 Taiwan
3Master Program of Food and Drug Safety, China Medical University, Taichung, 40402 Taiwan
4Institute of Toxicology, College of Medicine, National Taiwan University, No. 1, Jen-Ai Road, Section 1, Taipei, 10051 Taiwan
5Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan
6Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan
https://dx.doi.org/10.1186%2Fs13550-020-0597-3
Summary
Colorectal cancer (CRC) is the third most frequent occurring cancer in men and the second most frequent occurring cancer in women, with nearly 1.65 million new diagnosed cases and about 832,000 deaths in 2015. One possible cause of treatment failure is that the tumor site contains a small population of tumor-initiating cells termed cancer stem cells (CSCs). CSCs are involved in drug resistance, metastasis, and relapse of cancers, which can significantly affect tumor therapy. Hence, to develop specifically therapeutic target probe at CSCs for improvement of survival and quality of life of cancer patients is urgently needed. The CD166 protein has been suggested to be involved in CRC tumorigenesis and to be considered a marker for colorectal CSCs (CRCSCs) detection. In this study, therefore, the authors attend to apply a nuclear imaging agent probe, Glycine18-Cystine-linked CD166-targeted peptides (CD166tp-G18C), to detect the changes of CD166 level in a CRC xenograft mouse model.
Results from the nanoSPECT/CT
For the animal experiment, the authors have used a nanoSPECT/CT, which provided a good enough sensitivity and resolution to make the tumor uptake visible after 2 hours p.i., and see significant differences in the uptake of the applied tracers.
To create a xenograft tumor model, male BALB/c nude mice were subcutaneously inoculating CD166+HCT15 cells (1 × 106 cells) for 2 weeks, and then the 111In-DTPA, 111In-DTPA-G18C, 111In-DTPA-CD166tp-C, and 111In-DTPA-CD166tp-G18C (740 MBq/kg/mouse) were intravenously injected into mice. The imaging of CD166 in mice at 2, 4, 24, and 48 h were detected by the nanoSPECT/CT. For competitive study, the CD166+HCT15-derived xenograft mice were pre-treated with CD166tp-G18C (0, 10, and 50 mg/kg) for 6 h. Every mouse then received 740 MBq/kg 111In-DTPA-CD166tp-G18C via intravenous injection for 24 and 48 h. The competitive CD166 images were observed with the same procedure.
Figure 8. shows the main results from the SPECT/CT acquisitions: The nuclear imaging tracer of 111In-DTPA-CD166tp-G18C for detection of CD166-positive colorectal tumor in vivo. a) The colorectal tumor nuclear imaging analysis in CD166+HCT15 xenograft mice. The 111In-DTPA-CD166tp-G18C and control groups (740 MBq/kg/per mouse) were intravenously injected into mice for 2, 4, 24, and 48 h and detected by a nanoSPECT/CT. Group I, 111In-DTPA; Group II, 111In-DTPA-G18C; Group III, 111In-DTPA-CD166tp-C; Group IV, 111In-DTPA-CD166tp-G18C. b) Quantification of nuclear images in tumor areas of colorectal tumor xenograft mice. The circled positions in images were quantified by a 3D analysis software. Data are presented as mean ± SD (n ≥ 3). *P < 0.05, versus control group. c) The competitive study of 111In-DTPA-CD166tp-G18C in CD166+HCT15 xenograft mice. After tumor xenograft mice were intravenously injected with CD166tp-G18C (0, 10, and 50 mg/kg) for 6 h, 111In-DTPA-CD166tp-G18C (740 MBq/kg/mouse) was intravenously injected into mice for 24 and 48 h and detected by a nanoSPECT/CT. d) Quantification of nuclear images in tumor areas of colorectal tumor xenograft mice. Data are presented as mean ± SD (n ≥ 3). *P < 0.05, versus 0 mg/kg CD166tp-G18C group, #P < 0.05, versus 0 mg/kg CD166tp-G18C group.
- The authors have developed a nuclear imaging agent (111In-DTPA-CD166tp-G18C) using CD166tp-G18C as a probe for CD166-positive CRCs detection in a xenograft mouse model. In this xenograft model, when the tumor size achieved about 150 mm3 which possessed about 1 × 107 CD166-postive cells (cancer cell average diameter: 15 μm), the nanoSPECT/CT detection started to perform.
- These results suggest that CD166-positive CRC exhibited characteristics of CSCs, so it may be a useful drug screening tool for CRC diagnosis. The authors synthesized DTPA-CD166tp-G18C and radiolabeled with Indium-111 for detecting CD166 imaging by using nanoSPECT/CT in CD166-positive CRC xenograft mice. The bio-distribution of 111In-DTPA-CD166tp-G18C confirmed the accumulation of CD166-positive cells in tumors. Therefore, 111In-DTPA-CD166tp-G18C may be a potential nuclear imaging agent for diagnosis of CRCSCs. The CD166 bound peptide-based nuclear imaging may provide physicians to classify cancer cells before treatment and monitor patients with a history of CRC after surgery or drug treatment.

Synthesis, in vitro and in vivo evaluation of 11C-O-methylated arylpiperazines as potential serotonin 1A (5-HT1A) receptor antagonist radiotracers
Vidya Narayanaswami, Junchao Tong, Ferdinando Fiorino, Beatrice Severino, Rosa Sparaco, Elisa Magli, Flavia Giordano, Peter M. Bloomfield, Jaya Prabhakaran3, J. John Mann, Neil Vasdev, Kenneth Dahl and S. Dileep Kumar
![]() https://doi.org/10.1186/s41181-020-00096-8
https://doi.org/10.1186/s41181-020-00096-8
Peter M. Bloomfield and his colleague, Junchao Tong, from Centre for Addiction and Mental Health (CAMH), Toronto, Ontario have used Mediso nanoScan PET/MR 3T for testing candidate serotonin receptor radioligands in this publication.
Summary
Clinical importance of 5-HT1A receptors in the pathogenesis of several psychiatric and neurodegenerative disorders has promoted development of carbon-11 and fluorine-18 labeled radiotracers for in vivo positron emission tomography (PET). The gold standard PET imaging agent limited its widespread use.
The purpose of the current study was to develop and characterize a radioligand with suitable characteristics for imaging 5-HT1A receptors in the brain. The authors have reported the in vitro pharmacological characterization, radiosynthesis and preliminary in vivo PET imaging of three new 5-HT1A receptor arylpiperazine based ligands in rats (DF-100 (1), DF-300 (2) and DF-400 (3)).
They concluded DF-400 represents a promising O-methylated lead candidate which if subjected to structural alterations, may either lead to improved selectivity for 5-HT1A receptors or may assist in the development of the first PET radioligand for α1-adrenergic receptors.
Results from nanoScan PET/MRI 3T
- Dynamic PET studies in rats demonstrated negligible brain uptake of [11C] DF100 (1) and [11C] DF-300 (2). In contrast, significant brain uptake of [11C] DF400 (3) was observed.

Fig. 2 Uptake of [11C]3 (a); [11C]2 (b) and [11C]1 (c) in rat brain. Shown are TACs averaged for left and right brain (A: n = 3; B and C: n = 1) in SUV and summed (0–60 min) PET images in coronal, transverse and sagittal planes, respectively, through the thalamus. The spatially co-registered MR images (2D fast spin echo) show left-half ROIs including thalamus (blue), anterior cingulate cortex (red), hippocampus (green) and cerebellum (magenta) for the corresponding color-coded TACs
- Nevertheless, DF-400 displayed significant off-target binding attributed to α1-adrenergic receptors based on regional distribution (thalamus>hippocampus) and blocking studies

Fig. 2 Blocking of the uptake of [11C]3 in rat brain by WAY-100635 (a) and prazosin (b). Shown are TACs, averaged for left and right brain, (n = 1; solid: baseline; dashed: blocking) in SUV and summed (0–60 min) PET images in coronal, transverse and sagittal planes, respectively, through the thalamus at baseline and under blocking conditions. The three depicted left-half ROIs include thalamus (orange), hippocampus (red) and cerebellum (magenta) for the corresponding color-coded TACs
Preclinical Evaluation of a Novel 99mTc-Labeled CB86 for Rheumatoid Arthritis Imaging
Peng Liu1, Tingting Wang1, Rongshui Yang1, Wentao Dong1, Qiang Wang1, Zhide Guo2, Chao Ma1, Weixing Wang1, Huaibo Li1, and Xinhui Su1
1Department of Nuclear Medicine, Zhongshan Hospital Xiamen University, Xiamen 361004, China
2Center for Molecular Imaging and Translational Medicine, Xiamen University, Xiamen 361102, China
https://doi.org/10.1021/acsomega.0c04066
Summary
Early diagnosis and therapy are crucial to control disease progression optimally and achieve a good prognosis in rheumatoid arthritis (RA). Moreover, therapeutic intervention should start as soon as the diagnosis has been established, with the aim of stopping inflammation before irreversible damage is caused, but unfortunately current diagnostic methods are still not very sensitive and specific to RA.
Early hallmark of RA is the increased number of activated macrophages in the synovium with strong increase of their translocator protein (TSPO) level.
In previous studies a 99mtechnetium-labeled TSPO ligand (99mTc-CB256) was used to image a TSPO-rich cancer cell in vitro; however, few 99mTc-CB256 in vivo evaluation has been reported so far probably due to the cytotoxicity of CB256 (75 times more than analogous CB86). Here, a novel TSPO targeting radiopharmaceutical consisting of CB86 and diethylenetriaminepentaacetic acid (DTPA) is described.
Cytotoxicity, binding affinity and specificity of 99mTc-DTPA-CB86 to TSPO were evaluated using RAW264.7 macrophage cells. Biodistribution and 99mTc-SPECT studies were conducted on RA rat models after the injection of 99mTc-DTPA-CB86 with or without co-injection of unlabeled DTPA-CB86.
The probe displayed good stability in vitro and binding specificity to RAW264.7 macrophage cells. In the biodistribution studies, 99mTc-DTPA-CB86 exhibited rapid inflammatory ankle accumulation. At 180 min after administration, 99mTc-DTPA-CB86 uptakes of the left inflammatory ankle were 2.35 ± 0.10 percentage of the injected radioactivity per gram of tissue (% ID/g), significantly higher than those of the normal tissues. 99mTc-SPECT imaging studies revealed that 99mTc-DTPA-CB86 could clearly identify the left inflammatory ankle with good contrast at 30−180 min after injection. Therefore, 99mTc-DTPA-CB86 may be a promising probe for arthritis 99mTc-SPECT imaging.
Results from nanoScan SPECT/CT
The RA rats (n = 4 for each group) were injected with 99mTc-DTPA-CB86 (0.37 MBq, 100 μL) with or without co-injection of unlabeled DTPA-CB86 (300 μg) through the tail vein. At 30, 90, and 180 min after injection, they were anesthetized with 2% isoflurane and placed on the SPECT bed. SPECT acquiring parameters were as follows: a 140 keV energy peak for 99mTc, window width of 20%, a matrix of 256 × 256 and time frame 30 s. Whole-body static images (200 000 counts) were acquired with a matrix of 218 × 218, and a zoom of 2.0. CT data were acquired using an X-ray voltage biased to 50 kVp with a 670 μA current, with #projections 720°. Regions of interest (ROI) were drawn over the left inflammatory ankle and normal muscle, and then the ratios of the left inflammatory ankle to muscle were calculated.
- 99mTc-DTPA-CB86 accumulated in the left inflammatory ankles at 30 min and then showed a gradual increase of uptake. During 90−180 min after injection, the left inflammatory ankles were clearly visible, with good inflammatory to background contrast.
- When co-injected with unlabeled DTPA-CB86 (300 μg), the left inflammatory ankles were barely visible on SPECT images at 30−180 min after injection.
- Regions of interest (ROI) analysis of SPECT showed a high ratio of the left inflammatory ankle to muscle for RA rats injected unblocking dose compared to with 300 μg blocking dose at 30−180 min postinjection (P < 0.05).
- Evaluation of the probe in these RA rats demonstrated that 99mTc-DTPA-CB86 may be a promising agent for TSPO SPECT imaging.

Opioid–galanin receptor heteromers mediate the dopaminergic effects of opioids
Ning-Sheng Cai1, César Quiroz1, Jordi Bonaventura2, Alessandro Bonifazi3, Thomas O. Cole4, Julia Purks5, Amy S. Billing6, Ebonie Massey6, Michael Wagner6, Eric D. Wish6, Xavier Guitart1, William Rea1, Sherry Lam2, Estefanía Moreno7, Verònica Casadó-Anguera7, Aaron D. Greenblatt4, Arthur E. Jacobson8, Kenner C. Rice8, Vicent Casadó7, Amy H. Newman3, John W. Winkelman5, Michael Michaelides2, Eric Weintraub4, Nora D. Volkow9, Annabelle M. Belcher4, Sergi Ferré1
1Integrative Neurobiology Section.
2Biobehavioral Imaging and Molecular Neuropsychopharmacology Unit and.
3Medicinal Chemistry Section, National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP), NIH, Baltimore, Maryland, USA.
4Division of Alcohol and Drug Abuse, Department of Psychiatry, School of Medicine, University of Maryland, Baltimore, Maryland, USA.
5Massachusetts General Hospital, Departments of Psychiatry and Neurology, Harvard Medical School, Boston, Massachusetts, USA.
6Center for Substance Abuse Research, University of Maryland, College Park, Maryland, USA.
7Department of Biochemistry and Molecular Biomedicine, University of Barcelona, Barcelona, Spain.
8Drug Design and Synthesis Section, NIDA, IRP, and.
9NIDA, NIH, Baltimore, Maryland, USA.
https://doi.org/10.1172/jci126912
Summary
Identifying nonaddictive opioid medications is a high priority in medical science, but μ-opioid receptors (MORs) mediate both the analgesic and addictive effects of opioids. And as possibly everyone knows, the opioid epidemic shows a severe public health crisis worldwide.
Maintenance treatment with the (MOR) agonist methadone is the most highly researched and evidence-based treatment for opioid use disorder. Yet public perception concerning the substitution of illicit drugs (such as heroin) with medication (such as methadone) has led to stigmatized views of maintenance treatment, stalling the advancement of addiction treatment policy and access to medication-based treatments. MOR agonism also offers the most effective treatment for severe pain, making the search for a nonaddictive opioid drug the holy grail of pain research.
The neuropeptide galanin acts as a modulator of neurotransmission in the CNS and the PNS, and It is coexpressed with different neurotransmitters and coreleased by the major ascending noradrenergic, serotoninergic, histaminergic, and cholinergic systems. The authors recently reported the existence of functionally significant heteromers of the MOR and galanin 1 receptor (Gal1R) in the ventral tegmental area that could explain these galanin-opioid antagonistic interactions.
The present study sought to answer 2 main questions that arose from our study of MOR-Gal1R heteromers: (a) What are the mechanisms involved in the interactions between galanin and opioid ligands within the MOR-Gal1R heteromer? and (b) Do these interactions also involve morphine and synthetic opioids, such as methadone or fentanyl, differentially?
Results from nanoScan PET/CT
For the animal experiments, the authors have used a nanoScan PET/CT, which provided high resolution and sensitivity to follow the [18F]FDG uptake in the selected brain regions, and find significant differences after using the below mentioned drugs in rats.
The timeline of the experiment was slightly different from the usual PET/CT scans, as it involved two acquisitions: for the baseline scan, saline was injected i.p. (1 ml/kg) into the rats, and after 30 mins, [18F]FDG tracer was applied (i.p. as well). After 30 mins post-injection time, conventional 20 mins long PET/CT acquisitions was performed. After 2 days, the second part was coming, but instead of saline, morphine (1 mg/kg) or methadone (1 mg/kg) was injected i.p. After accessing [18F]FDG, the second PET/CT was conducted as before. For the reconstruction, Teratomo 3D engine was used with attenuation and scatter corrections, with a 0.4 mm resolution.
Figure 4. shows the main results from the PET/CT acquisitions: A. The timeline of the experiment (explained above). B. [18F]FDG uptake after administration of saline (baseline, n = 14), morphine (1 mg/kg, n = 7), or methadone (1 mg/kg, n = 7). Coronal and sagittal images (1.5 mm anterior to bregma and 1.4 mm lateral from the midline, respectively) show the average SUVR calculated using the whole brain as a reference region. C. Voxel-based parametric mapping analyses revealed significantly decreased metabolic activity from baseline values in a basal forebrain region that included the nucleus accumbens and its projecting areas after morphine, but not methadone, treatment. Statistical parametric maps of significant decreases of [18F]FDG uptake (P < 0.05, paired t test). D. and E. VOI analyses of the frontal cortex (FCx), dorsal striatum (DS), and basal forebrain (BF) region, showing a significant differential pattern of [18F]FDG uptake after administration of morphine (D) or methadone (E).
- The authors found significant pharmacodynamic difference between morphine and methadone that is determined entirely by heteromerization of MORs with Gal1Rs, rendering a profound decrease in the potency of methadone. This finding was explained by the weaker proficiency of methadone in activating the dopaminergic system as compared with morphine and predicted a dissociation of the therapeutic and euphoric effects of methadone, which was corroborated by a significantly lower incidence of self-reports of feeling “high” in methadone-medicated patients.
- These results suggest that μ-opioid–Gal1R heteromers mediate the dopaminergic effects of opioids. The results further suggest a lower addictive liability of some opioids, such as methadone, due to their selective low potency for the μ-opioid–Gal1R heteromer.

PET imaging of P2X7R in the experimental autoimmune encephalomyelitis model of multiple sclerosis using [11C]SMW139
https://doi.org/10.1186/s12974-020-01962-7
Summary
Neuroinflammation plays a central role in a variety of pathologies affecting the central nervous system (CNS), such as multiple sclerosis (MS), Alzheimer’s and Parkinson’s disease. Microglia are major contributor in disease’s pathogenesis, although the exact role of microglia and their activation status during the disease process is not understood exactly.
In this article the process of neuroinflammation has been studied in Lewis rats with experimental autoimmune encephalomyelitis (EAE), an animal model for MS.
Mediso nanoScan PET/CT and nanoScan PET/MRI were used for non-invasive imaging of the activation status of microglia and the ability to identify a pro- or anti-inflammatory environment.
The authors have used a C-11 isotope labelled purinergic receptor (P2X7R) ligand ([11C]SMW139) for tracing microglial activity. They assessed the tracer’s potential for imaging neuroinflammation and its specific binding to P2X7R. They also matched the molecular imaging result with autoradiography and immunohistochemistry.
The authors have shown that [11C]SMW139 is a promising PET tracer for imaging neuroinflammation and evaluating the dynamics of pro-inflammatory microglia in the brain.
Selected results from nanoScan PET/MRI and nanoScan PET/CT
- They evaluated the uptake of [11C]SMW139 at the peak of inflammation and compared it to the uptake in the recovery phase.

Fig 1. Sagittal PET images extracted from the static reconstruction of the 5–45 min frame and showing [11C]SMW139 uptake in the brain and spinal cord (arrows) of severe-relapsing (a), severe acute (b). Arrow heads are showing [11C]SMW139 uptake in a brain draining lymph node

Fig 2. Sagittal PET images extracted from the static reconstruction of the 5–45 min frame and showing [11C]SMW139 uptake in the brain and spinal cord (arrows) of relapsing EAE rat (peak of the disease (e), relapse (f), recovery (g)) and non-relapsing rat ( of the disease (h), no-relapse (i), recovery (j)). S.C. spinal cord, CB cerebellum, B.S. brain stem. Data are expressed as percent injected dose per milliliter (%ID/mL)
- They validated the specificity of [11C]SMW139 tracer binding to the EAE tissue

Fig 3. Correlation between uptake of [11C]SMW139 tracer in the brain of the EAE animal and the ex vivo immunostaining for IBA-1 and ED-1; Transversal (A) and sagittal (D) PET image section showing the uptake of the [11C]SMW139 in the brain. The dotted purple circles or rectangles mark the area with the highest uptake. The green and red spots in the brain indicate a high accumulation of [11C]SMW139; Immunostaining with IBA-1 (B, E) and CD68 (C, F) of the respective brain region post PET imaging showing high microglia activation in the same region where the high uptake of [11C]SMW139 was observed by PET imaging.
Comparison of SARS-CoV-2 infection in two non-human primate species: rhesus and cynomolgus macaques
Kinga P. Boszormenyi1, Marieke A. Stammes2, Zahra C. Fagrouch1, Gwendoline Kiemenyi-Kayere1, Henk Niphuis1, Daniella Mortier1, Nikki van Driel3, Ivonne Nieuwenhuis2, Ella Zuiderwijk-Sick4, Lisette Meijer2, Petra Mooij1, Ed J. Remarque1, Gerrit Koopman1, Alexis C. R. Hoste5, Patricia Sastre5, Bart L. Haagmans6, Ronald E. Bontrop7,8, Jan A.M. Langermans3,9, Willy M. Bogers1, Ernst J. Verschoor1, and Babs E. Verstrepen1
1Department of Virology, Biomedical Primate Research Centre (BPRC), Rijswijk, The Netherlands
2Department of Parasitology, BPRC, Rijswijk, The Netherlands
3Animal Science Department, BPRC, Rijswijk, The Netherlands
4Alternatives unit, BPRC, Rijswijk, The Netherlands
5Eurofins-Inmunologia y Genetica Aplicada (Eurofins-INGENASA), Madrid, Spain
6Department of Viroscience, Erasmus University Medical Center, Rotterdam, The Netherlands
7Department of Comparative Genetics and Refinement, BPRC, Rijswijk, The Netherlands
8Department of Biology, Theoretical Biology and Bioinformatics, Utrecht University, Utrecht, The Netherlands
9Department of Population Health Sciences, Unit Animals in Science and Society, Veterinary Faculty, Utrecht University, Utrecht, The Netherlands
https://doi.org/10.1101/2020.11.05.369413
Summary
SARS-CoV-2 is a coronavirus that sparked the current COVID-19 pandemic. To stop it, effective and safe vaccines, and antiviral therapies are urgently required. To facilitate the preclinical evaluation of intervention approaches, relevant animal models need to be developed and validated. Rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis) are widely used in biomedical research and serve as models for SARS-CoV-2 infection, but this is the first controlled comparative study investigating which species of them is best suited to examine specific aspects of COVID-19. This study analysed replication and symptoms for three weeks after infection. Pulmonary lesions were detected on CT images acquired with MultiScan LFER PET/CT. Elevated body temperature and decreased in physical activity was also observed. Results show that both rhesus and cynomolgus macaques represent valid models for COVID-19 prophylactic and therapeutic treatments.
Results from MultiScan LFER PET/CT
CT imaging provides a valuable tool to specifically monitor the progression of COVID-19-related lung pathology during the entire course of the study. Respiratory-gated CT scans were performed on Day0, 2, 4, 6, 8, 10, 12, 14, 16, 22 post-infection to monitor lung pathology. A semi-quantitative scoring system for chest CT evaluation was used to estimate SARS-CoV-2-induced lung disease; maximum score of 35 could be reached per timepoint.
Scans revealed that:
- All macaques show levels of pneumonia
- Detected different types of lesions: A) ground glass opacities, B) consolidations, and C) crazy paving patterns (Figure 1)
- Around days 8 and 10 pi., lesions were manifest in all animals, and in several macaques the coverage had increased
- Cumulative CT scores increased and no difference was observed between rhesus and cynomolgus macaques

Further results showed:
- Both groups of animals, the body temperature was significantly higher during the first two weeks after infection
- Significantly lower activity in all four rhesus macaques during the first period after infection, while this difference in cynomolgus macaques was less obvious
- Antibody response became evident between day 10 and 12 pi., IgG level continued to rise for several days (development of IgM titers was barely detected)
- Certain cytokines increased in the plasma of both macaque species
Heterodimeric Radiotracer Targeting PSMA and GRPR for Imaging of Prostate Cancer-Optimization of the Affnity towards PSMA by Linker Modification in Murine Model
Fanny Lundmark1, Ayman Abouzayed1, Bogdan Mitran1,2, Sara S. Rinne1, Zohreh Varasteh1,3, Mats Larhed4 , Vladimir Tolmachev5,6 , Ulrika Rosenström1 and Anna Orlova 1,4,6
1 Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
2 Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet and Stockholm County Council, 171 77 Stockholm, Sweden
3 Department of Nuclear Medicine, Klinikum rechts der Isar der TUM, 80802 Munich, Germany
4 Science for Life Laboratory, Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden
5 Department of Immunology, Genetics and Pathology, Uppsala University, 751 83 Uppsala, Sweden
6 Research Centrum for Oncotheranostics, Research School of Chemistry and Applied Biomedical Sciences, Tomsk Polytechnic University, 634050 Tomsk, Russia
doi:10.3390/pharmaceutics12070614
Summary
Prostate-specific membrane antigen (PSMA) and gastrin-releasing peptide receptor (GRPR) are overexpressed in prostate cancer (PCa) cells and are promising targets for molecular imaging methods used for diagnosis. Novel heterodimer - containing PSMA inhibitor and GRPR antagonist - has been demonstrated to bind specifically to both proteins with concomitant low uptake in normal tissues. In the current study, chemical structure of the heterodimer was modified in order to improve affinity towards PCa cells and binding characteristics were analysed. Tumor-bearing mice were injected with 111In-labeled heterodimer (BQ7812). In vivo biodistribution was investigated on harvested organs and also with SPECT/CT imaging. Quantitative analysis together with in vitro tests revealed that modifications in the molecular design resulted in 10-fold improved affnity towards PSMA and high activity uptake in tumors.
Results from nanoScan SPECT/CT
For the SPECT/CT studies, BALB/c nu/nu mice implanted with PC3-pip (isogenic human prostate carcinoma) cells were injected with 830kBq 111In-BQ7812. Groups were also co-injected with non-labeled GRPR antagonist and/or non-labeled PSMA-11 to block GRPR and/or PSMA to prove binding specificity. Imaging of the non-blocked group was performed at 1 and 3h pi and for the GRPR/PSMA-blocked group at 1h pi.
Result revealed that:
- images are in good agreement with the ex vivo analysis: tumor could be visualized already at 1h pi. and the only healthy organs with high activity uptake at this time point were the kidneys (Figure 1.A)
- Co‐injection of non-labeled PSMA-11 and NOTA-PEG4-RM26 resulted in a decreased kidney uptake and a negligible activity uptake in the tumor (Figure 1.B)
- activity cleared from healthy organs and blood with time, leading to an improved imaging contrast at 3h pi. (Figure 1.C)
- ∑ Together with the results from the in vitro and in vivo specificity tests, confirmed the specific binding of [111In]In-BQ7812 to both PSMA and GRPR

Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics
Oskar Vilhelmsson Timmermand1, Jörgen Elgqvist2, Kai A. Beattie3, Anders Örbom1, Erik Larsson4, Sophie E. Eriksson1, Daniel L.J. Thorek5, Bradley J. Beattie6, Thuy A. Tran7,8, David Ulmert1,3,9, Sven-Erik Strand1,4
1Division of Oncology and Pathology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
3Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
4Division of Medical Radiation Physics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
5Department of Radiology, Washington University School of Medicine, Saint Louis, MO, 63108, USA
6Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
7Department of Radiopharmacy, Karolinska University Hospital, Stockholm, Sweden
8Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
9Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at University of California, Los Angeles (UCLA), CA, USA
https://doi.org/10.7150/thno.31179
Summary
Particular metastatic prostate cancers can be treated well with androgen ablating drugs, however resistance is probably developing, and with the increased expression of the Androgen Receptor (AR), the tumor may growths back.
Human Kallikrein 2, which is a downstream molecule of AR pathway, can be a potential target, and the authors have used an antibody (hu11B6) against it. They assessed the efficacy of hu11B6 labeled with a low LET beta-emitter, Lutetium-177 (177Lu) and investigated whether similar tumor killing and AR-enhancement is produced. Moreover, single-photon emission computed tomography (SPECT) imaging of 177Lu is quantitatively accurate and can be used to perform treatment planning. [177Lu]hu11B6 therefore has significant potential as a theranostic agent.
Results from nanoSPECT/CT Plus
For the SPECT/CT studies, the authors have used a nanoSPECT/CT Plus, which is a precise option to follow the biodistribution of 177Luhu11B6, and follow the tumor size in mice with good resolution. Subcutaneous PCa xenografts (LNCaP s.c.) were grown in male mice. Biokinetics at 4-336 h post injection and uptake as a function of the amount of hu11B6 injected at 72 h were studied. Over a 30 to 120-day treatment period the therapeutic efficacy of different activities of [177Lu]hu11B6 were assessed by SPECT/CT imaging besides other options.
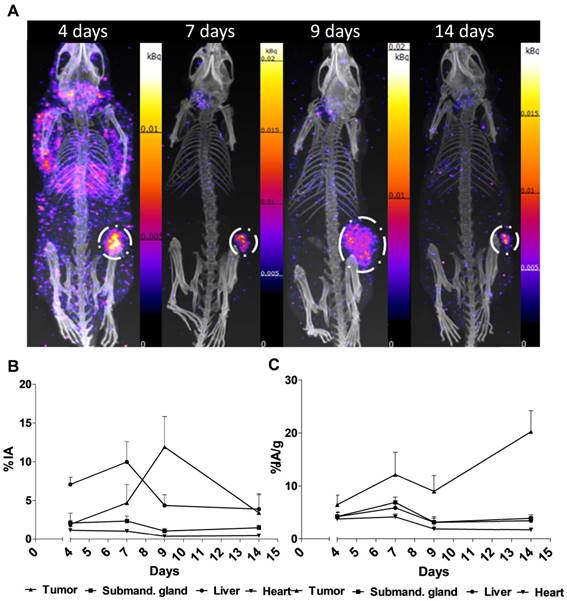
Performing the acquisitions, a multipinhole mouse collimator was used and with energy windows of 20% centered over the 56-, 113-, and 208-keV energy peaks of 177Lu. Acquisition time was about 40 min. With the help of the CT images as an anatomical reference, regions of interest (ROI), where drawn for tumor, submandibular glands, liver and heart.
Figure 2. shows the main results from the SPECT/CT acquisitions: A. Representative maximum intensity projections of SPECT/CT of mice at 4, 7, 9 and 14 days p.i. of 177Lu-hu11B6. B. Biokinetics as percent injected activity per gram of tissue (%IA/g) of therapeutic amounts of 177Lu-hu11B6 (20-30 µg) quantified from SPECT data. Quantified data from SPECT/CT imaging of 177Lu-hu11B6 for tumor, submandibular gland, liver and heart. C. Quantified %IA data from SPECT/CT imaging of 177Lu-hu11B6.
- The results suggest tumor accumulation of [177Lu]hu11B6 peaked at 168 h with a specific uptake of 29 ± 9.1 percent injected activity per gram (%IA/g) and low accumulation in normal organs except in the submandibular gland (15 ± 4.5 %IA/g), attributed to a cross-reaction with mice kallikreins in this organ, was seen. However, SPECT imaging with therapeutic amounts of [177Lu]hu11B6 revealed no peak in tumor accumulation at 7 d, probably due to cellular retention of 177Lu and decreasing tumor volumes.
- This study shows that hu11B6 labeled with the low LET beta-emitting radionuclide 177Lu can deliver therapeutic absorbed doses to prostate cancer xenografts with transient hematological side-effects.
Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo.
Elashiry, M. et al., Journal of Extracellular Vesicles 9, 1795362 (2020).
doi: 10.1080/20013078.2020.1795362
Summary
This recently published study is the first demonstration of DC exosome-based therapy for a degenerative alveolar bone disease and provides the basis for a novel treatment strategy.
Periodontitis (PD) is a chronic bone disease that affects over 50% of the U.S. population. Severe PD lesions are infiltrated with B cells, macrophages, and dendritic cell (DC) clusters with CD4+T cells. The immune response can be shaped based on the maturation status of DCs, yet no effective immunomodulatory agent for PD has been identified. The goal of the current study was to characterize the immunobiology of DC derived exosome subtypes in vitro and in vivo and their ability to reprogram immune cells responsible for inflammatory bone loss.
The authors have used nanoScan SPECT/CT for imaging of exosome biodistribution in the murine periodontitis model.
Results from nanoScan SPECT/CT
Locally administrated exosomes showed higher affinity and slower clearance from periodontal tissues in the inflammatory, alveolar bone loss model. (A) SPECT CT live animal in vivo imaging of free, In-111 (left) or In-111-labelled, exosomes (right) in mice after the 24h IV administration. (B) Local delivery of free, In-111 (left) or In-111-labelled, exosomes (right) by injection in the palatal gingiva at the right side of maxilla was utilized. (C) Radioactivity in maxilla, relative to the total, when free or bound to DC EXO, was expressed as a percentage determined by using SPECT CT images. (D) Radioactivity in maxilla, relative to the total, when free or bound to DC EXO, was expressed as a percentage, in post-mortem isolated maxilla, determined by a gamma counter. Mice were subjected to ligature placement to induce inflammatory bone loss prior to imaging. Yellow arrows delineate maxilla, white arrows liver, spleen and other non-oral sites.

Preclinical evaluation of an 111In/225Ac theranostic targeting transformed MUC1 for triple negative breast cancer
Vanessa J Kelly1, Shu-Ta Wu2,3, Vijay Gottumukkala1, Richard Coelho1, Keryn Palmer1, Surabhi Nair1,
Timothy Erick2, Rahul Puri3, Ohad Ilovich1, Pinku Mukherjee2,3
- Invicro, LLC, Boston, MA, USA
- Department of Biological Sciences, University of North Carolina, Charlotte, NC, USA
- OncoTAb, Inc., Charlotte, NC, USA
Summary
Triple-negative breast cancers (TNBC) are associated with poor prognosis and high mortality rates following relapse. These cells do not express estrogen, progesterone or HER2/neu receptors, which means that receptor targeted therapies can’t be utilized. These cells overexpress transformed MUC1 (tMUC1) antigen, which is selectively bound by murine antibody TAB004. Once TAB004 binds to tMUC1 it has been shown to internalize, which makes them excellent therapeutic candidate for TNBC.
Current study aimed to evaluate humanized TAB004 (hTAB004) as a potential theranostic for TNBC. hTAB004 was labeled either with Indium-111 (for biodistribution analysis) or Actinium-225 (for alpha radiotherapy) and injected intravenously to orthotopic tumor bearing mice. Results demonstrate both high tumor concentrations and high tumor-to-blood ratios. Additionally, a single administration of 225Ac-DOTA-hTAB004 increased survival and resulted in consistently lower tumor volumes compared to the control group after 12 days. Together they are convincing proof-of-concept support for hTAB004 as a theranostic agent in triple negative breast cancer.
Results from nanoScan SPECT/CT
1) In vivo biodistribution studies: NSG mice were inoculated with HCC70 tumors. 27 days later 7.5MBq 111In-DOTAhTAB004 was intravenously injected via the tail vein. SPECT-CT imaging was performed at 4, 24, 48 and 120 h postinjection.
The tumor accumulation of 111In-DOTA-hTAB004 increased over 120 h reaching a maximum of 65.4 ± 15.2 %ID/g. All other organs (blood, bone, kidneys, liver, lungs, muscle, pancreas, spleen) had <10% ID/g at this time.
2) Efficacy studies: Athymic nude mice were inoculated with HCC70 tumor cells. 27 days later they were injected via the tail vein with either 225Ac-DOTA-hTAB004 (18.5 kBq) or DOTA-hTAB004 and monitored for tumor growth via caliper measurements. The 225Ac-DOTA-hTAB004 group had significantly smaller tumors and greater survival compared to the control group.

18F-FDG-PET Detects Drastic Changes in Brain Metabolism in the Tg4–42 Model of Alzheimer’s Disease
Caroline Bouter1, Philipp Henniges2, Timon N. Franke2, Caroline Irwin2, Carsten Oliver Sahlmann1, Marius E. Sichler2, Nicola Beindorff3, Thomas A. Bayer2 and Yvonne Bouter2
1Department of Nuclear Medicine, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
2Division of Molecular Psychiatry, Department of Psychiatry and Psychotherapy, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
3Berlin Experimental Radionuclide Imaging Center (BERIC), Charité—University Medicine Berlin, Berlin, Germany
https://doi.org/10.3389/fnagi.2018.00425
Summary
The authors have used one of the latest mouse Alzheimer’s Disease (AD) models (Tg4-42 transgenic mutation, which overexpresses the Ab4-42 peptide, which is truncated on the N-terminal region, causing neurotoxicity and Ab aggregation, which are similar to AD). As the AD patients show altered glucose metabolism, the authors chose to follow it with the most common radiopharmaceutical used in PET, namely 18F-FDG, and how it can be used together with the MRI as an early biomarker for AD.
They have found that Tg4-42 mice show a reduction of glucose-metabolism, which correlates with their age, and the decreased 18F-FDG uptake can be shown in early age (3 months).
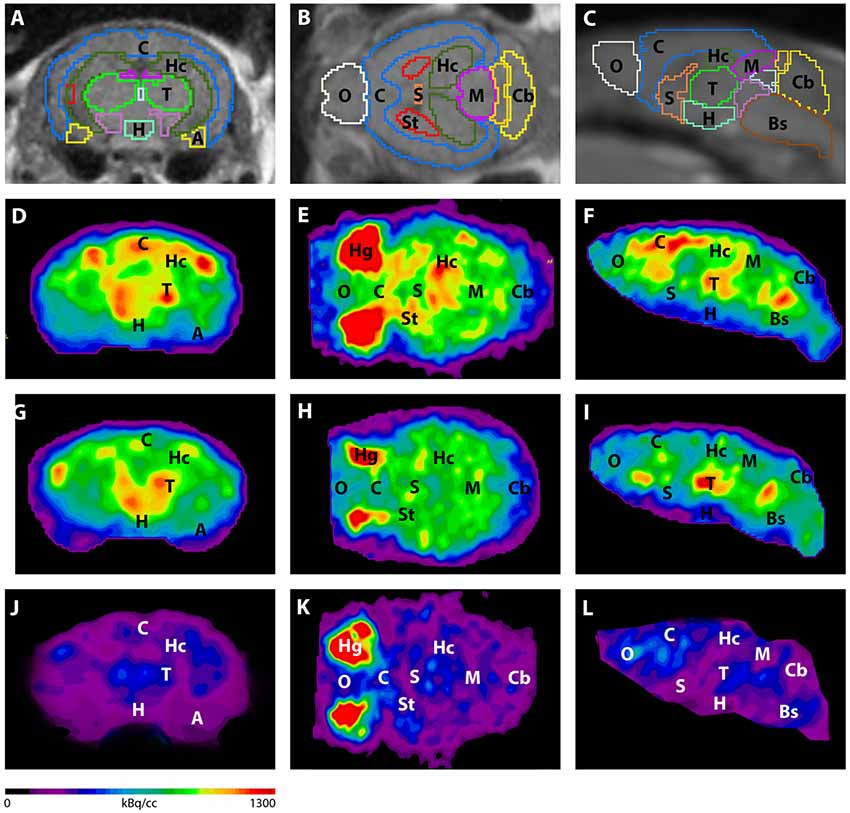
Results from nanoScan PET/MRI 1T
For the PET/MRI studies, the authors have used a nanoScan PET/MRI 1T, which could provide a fast measurement method together with good statistics. Young (3-4 months) and aged (7-8 months) Tg4-42 (n=7, female) and aged WT C57Bl/6J (7-8 months, n=5, female) control mice were used in this study. The authors have followed the standard 18F-FDG PET/MRI protocol, as the mice were fasted overnight, and 9-21 MBq activity was injected into the tail vein, with a 45 minute long uptake period.
The PET scans were performed for 20 minutes , which was followed by a Tera-Tomo 3D reconstruction method with a 0.3 mm3. For the MRI, the authors have used GRE sequence as a material map for attenuation and scatter correction in the PET reconstruction, and as a brain atlas. The analysis was performed with PMOD software.
Figure 3. shows the main results from the PET/MRI acquisitions: a-c) MRI images were matched with predefined brain regions; axial, coronal and sagittal view. d-f) 18F-FDG-PET images of a WT mouse. g-i) 18F-FDG-PET images of a young Tg4–42 mouse. j-l) 18F-FDG-PET images of an aged Tg4–42 mouse. A, Amygdala; Bs, Brain Stem; C, Cortex; Cb, Cerebellum; H, Hypothalamus; Hc, Hippocampus; Hg, Harderian glands; M, Midbrain; O, Olfactory Bulb; S, Septum/Basal Forebrain; St, Striatum; T, Thalamus.
- These results suggest that 18F-FDG uptake was distinctly lower in aged Tg4–42 mice compared to WT mice. In young Tg4–42 mice 18F-FDG the uptake did not show significant differences in whole brain uptake but it was reduced in the hippocampus, forebrain, hypothalamus, amygdala and midbrain.
- The study showed that Tg4-42 can be a useful AD model to monitor the effects of various therapeutic strategies in vivo using 18F-FDG uptake as a therapeutic readout.
Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model
Mudan Cai1, Jun-Hwan Lee2 and Eun Jin Yang2
1Department of Herbal Medicine Research, Korea Institute of Oriental Medicine, 1672 Yuseong-daero, Yuseong-gu, Daejeon, 305-811, Republic of Korea
2Department of Clinical Research, Korea Institute of Oriental Medicine, 1672 Yuseong-daero, Yuseong-gu, Daejeon, 305-811, Republic of Korea
https://doi.org/10.1186/s12974-019-1665-3
Summary
Current study investigated the electroacupuncture (EA)-induced molecular mechanisms causing cognitive improvement and anti-inflammatory activity in 5XFAD mice, a transgenic animal model of Alzheimer’s Disease (AD). Mice were bilaterally treated with EA three times per week for 2 weeks, thereafter Y-maze tests, western blots, immunohistochemistry, and PET scans were performed. Results revealed that EA treatment significantly improved working memory and synaptic plasticity, activated cell metabolism in the frontal cortex and the hypothalamus, concomitantly reduced neuroinflammation, ultrastructural degradation of synapses, and the microglia-mediated amyloid β deposition.
Results from nanoScan PET/CT
For the PET/CT studies, 7.4MBq 18F-FDG was intravenously injected via the tail vein to the following treatment groups: a) non-transgenic (non-Tg), b) 5XFAD (Tg) and c) EA-treated 5XFAD (Tg+KI3). After 60min uptake period, 30min long PET acquisitions were performed. Data were reconstructed with Tera-Tomo 3D reconstruction method. For quantitative analysis regions of interest were drawn into the frontal cortex, cortex, hippocampus, and hypothalamus and standardized uptake values (SUV) were evaluated to determine the effect of EA treatment on brain metabolism. Result revealed that:
-
EA stimulation caused a 1.1-fold increase in the mean glucose level of the frontal cortex, which is related to short-term and working memory showing that it improves cognitive functions
-
The hypothalamus, which is related to energy metabolism, exhibited a 1.1-fold decreased glucose metabolism in Tg mice compared to non-Tg mice, and this effect was fully reversed in EA-treated Tg mice proving that it modulates the abnormal hypothalamic metabolism related to the amyloid pathology in AD mice


Introduction
We would like to introduce Professor Ali Arbab and his colleagues’ work in this blog. Professor Ali Arbab is the leader of the Tumor Angiogenesis Initiative, and director of the Core Imaging Facilities for Small Animals (CIFSA) in Augusta, GA. His research group interests are antiangiogenic therapy and vascular mimicry in glioblastoma (GBM) and breast cancer, targeting the tumor microenvironment of GBM and breast cancers as a better option to counter therapy resistance. Recently they have explored engineered exosomes as imaging and therapeutic probes to target immune-suppressive cells in tumor microenvironment. Professor Ali Arbab has 30 years of experience in imaging science involving MRI, SPECT, CT, ultrasound, and optical imaging modalities. The core facility provides MRI and optical imaging resources, and they also have a nanoScan SPECT/CT from Mediso.
Publication
 |
Rashid, M. H. et al. Generation of Novel Diagnostic and Therapeutic Exosomes to Detect and Deplete Protumorigenic M2 Macrophages. Advanced Therapeutics 3, 1900209 (2020). |
Exosomes have emerged as potential tools for a drug delivery system that can target specific tissues or cells. M2 macrophages participate in immune suppression, epithelial to mesenchymal transition, invasion, angiogenesis, tumor progression, and subsequent metastasis foci formation. In their recent work they determined in vivo distribution of M2 macrophages with 111In-oxine-based radiolabeling of the targeted exosomes. The research demonstrated that M2 macrophage targeting therapeutic exosomes deplete M2 macrophages both in vitro and in vivo, and reduce tumor burden, increasing survival in a metastatic breast cancer model.
nanoScan SPECT/CT was used to detect the biodistribution of 111In-oxine-labeled exosomes targeting CD206-positive M2 macrophages.

Figure 4.d from the publication, After 3 h of intravenous injection showed significant accumulation of M2-targeting exosome in tumor, lung, spleen, lymph node, and bones. 111In-oxine-labeled non-targeting exosomes (HEK293 exo) and CD206-positive M2 macrophage targeting exosomes (M2-targeting exo) were injected into the 4T1 tumor bearing mice. One group was treated with Clophosome to deplete macrophages. Yellow and green arrows denote lymph node and bone metastasis, respectively.
Southwest National Primate Research Center in Texas Biomedical Research, San Antonio has purchased Mediso’s multiscan LFER PET/CT scanner in early 2020. Mediso USA proudly post that Professor Deepak Kaushal and his co-workers used multiscan LFER PET/CT in their recently reported comprehensive work about the course of SARS-CoV-2 infection in nonhuman primate models.
multiscan LFER PET/CT features a 20 cm axial and 15 cm transaxial field of view with a 26 cm bore opening which allows the researcher to scan non-human primates (NHP) with sub-mm PET and even 150 um CT resolution.
preliminary report title: SARS-CoV-2 infection leads to acute infection with dynamic cellular and inflammatory flux in the lung that varies across nonhuman primate species June 5, 2020. https://doi.org/10.1101/2020.06.05.136481
Summary
The authors compare SARS-CoV-2 infection in three species of experimentally infected NHPs (rhesus macaques, baboons, and marmosets). They used a wide variety of methods to describe the course of disease such as clinical parameters of viral infection, viral RNA and viral protein detection, immune response, X-ray, CXR scoring, CT scanning and pathology.
Their results show all NHPs can be infected with SARS-CoV-2 but exhibit differential progression to COVID-19. Baboons exhibit moderate to severe pathology, macaques exhibit moderate pathology and marmosets exhibit mild pathology. They also summarize that rhesus macaques and baboons develop different, quantifiable disease attributes making them immediately available essential models to test new vaccines and therapies.
Results from multiscan LFER PET/CT
The report shows the importance of state-of-the-art, non-invasive imaging – cone beam CT scanning, and the application of innovative algorithms to identify the extent of lung involvement in pneumonia in developing models of COVID-19. CT image analysis provided a quantifiable metric data which enables testing efficacy of vaccines or the impact of therapeutic intervention. Lung hyperdensity and the CT abnormality score was used as a metric parameter to follow the onset of the disease.
- Each NHP species was infected and followed over a 3-day period to describe the early signs of infection. Cone-beam CT scans showed evidence of moderate pneumonia, which progressed over 3 days (Figure 1). CT images were analyzed using the segmentation tool in VivoQuant (Invicro, Boston,MA). Lung hyperdensity and the CT abnormality score were used as a metric parameter to follow the course of the disease.

Figure 1. a) 3D Reconstruction of ROI volume representing the location of the lesion. b-d) represent images for quantification of the lung lesion with the green area representing normal intensity lung voxels (-850 HU to -500 HU), while red areas represent hyperdense voxels (-490 HU to 500 HU). (Image courtesy of Dr. Deepak Kaushal, Texas Biomedical Research)
- They also performed detailed imaging of macaques in a 12-day longitudinal study. Similar to the acute study. imaging revealed a significant, progressive increase in the volume of lung involved in pneumonia at 6 dpi, which normalized by 12 dpi.
- These results suggest that pneumonia in some older macaques may persist longer than in younger animals. Although there were several smaller changes observed in older animals, old and young animals both resolved the infection.
Introduction:
Small Animal Molecular imaging of infectious diseases in lung is becoming more and more important, especially at the time of COVID-19 outbreak. Dr. Sanjay K. Jain and his research group in Center for Infection and Inflammation Imaging Research (Ci3R, John Hopkins University) focuses on studying the pathogenesis of bacterial diseases, with a major focus on tuberculosis (TB). They have developed a pipeline of bacteria-specific PET-based imaging tracers. They also have developed imaging techniques to noninvasively determine antimicrobial penetration into infected lesions and understand lesions-specific host-responses. They translate their preclinical findings to the clinic and are testing some novel imaging tracers developed in their laboratory in human studies. For pre-clinical molecular imaging technique they are using Mediso nanoScan PET/CT, so they are able to follow kinetic changes of a drug distribution in high temporal resolution, and can locate sites of pathogen and inflammation in high spatial resolution.
The main challenge working with M. tuberculosis, and working with SARS-CoV-2 even, is that it requires Biosafety Level 3 (BSL-3) environment. Dr. Jain and his co-workers have developed sealed biocontainment chambers for mouse and rabbit compliant with BSL-3 requirements in house. The high versatility and open design of nanoScan PET/CT allowed them to install their chamber on the Mediso system. Working in a BSL-2 environment with a BSL-3 compatible chamber makes researcher’s life easier. For these reasons Mediso in collaboration with Dr. Jain has developed a commercially available BSL-3 compatible chamber for the PET/CT MultiCell™ imaging system, (see details below).
Clinical study findings supported by small animal imaging on Mediso nanoScan PET/CT
Ordonez et al. “Dynamic Imaging in Patients with Tuberculosis Reveals Heterogeneous Drug Exposures in Pulmonary Lesions.” Nature Medicine 26, no. 4 (April 2020): 529–34. (https://doi.org/10.1038/s41591-020-0770-2)
In this recent publication Ordonez et al. provided estimates on rifampin dosing, a first-line TB drug, required to achieve faster cure in 4 months. Newly identified patients were enrolled in a first-in-human study using dynamic [11C]rifampin positron emission tomography (PET) and computed tomography (CT). The findings from human studies were supported with small animal imaging using Mediso nanoScan PET/CT (extended data in publication Fig. 6).
An interesting finding was that [11C]rifampin exposures in human pulmonary TB lesions were low, were spatially compartmentalized and demonstrated between-patient and within-patient variability. [11C]rifampin (tissue-to-plasma) AUC ratios demonstrate limited [11C]rifampin exposure in lesions, with the lowest exposure noted in cavity walls, which paradoxically also have the highest bacterial burden. The pharmacokinetic studies conducted on a rabbit model also demonstrated limited and spatially compartmentalized [11C]rifampin exposures in TB lesions, with the lowest exposures in cavity walls.
The authors hypothesize that tissue necrosis and presumably the fibrotic extracellular matrix surrounding cavitary tissues limited the ability for passive diffusion and rifampin penetration into these lesions, thus minimizing the spread.
Commercially available BSL-3 compliant imaging chamber from Mediso
Mediso has developed in collaboration with Dr. Sanjay K. Jain a commercially available BSL-3 compliant imaging chamber for MultiCell™ imaging system.
The advance of using this chamber is that the PET/CT scanner doesn’t have to be in BSL-3 laboratory, so the animal imaging and the scanner maintenance become much easier. Moreover, the scanner in a such setup can be used for non BSL-3 required animal studies also.
As a workflow, the animals are anesthetized in a BSL-3 environment and placed into the imaging chamber and then sealed. The outer surface of the chamber is decontaminated before caring to BSL-2 environment.
The chamber can be attached by one-click to the scanner’s docking stage without any further tube connection. The anesthesia gas flows in and out through a 0.3 um pore filter. The heating air is circulating only in the chamber’s wall, so there is no transit between the heating air compartment and the chamber space. For kinetic studies the animal is cannulated in BSL-3 and the tubing is led out through a sealed opening (see Figure 1.)
This practical solution provides a tenable and flexible mechanism for routine imaging and for implementing the more severe constraints necessitated by BSL-3 imaging procedures in a simple and expeditious manner.

Figure 1. MultiCell™ BSL-3 compliant imaging chamber
A study by researchers at Huntsman Cancer Institute (HCI, Salt Lake City, Utah) has been recently published in the journal ‘Nature Medicine’. The paper proposes a new therapeutic approach for patients suffering from pancreatic cancer that may be effective to treat the disease. It involves a combination drug therapy and has been studied in vitro, in vivo also in a human volunteer.
As the new treatment combines two drugs that are both registered for use by the Food and Drug Administration, the clinical trial (‘THREAD’) is now open at HCI soon to be followed by other US sties.
The novel method involves targeting two physiological process at the same time. Previous studies have focused on only one process and shown to be ineffective. One process is an impact of a mutation in a gene called ‘KRAS’ that sends constant signals that promote cells divisions resulting in uncontrolled tumour growth. Another process, autophagy, is a cell-level recycling of cells including pancreatic cancer cells.
The new HCI study used mouse models to investigate a drug response of combining the two drugs simultaneously and applied advanced imaging techniques (nanoScan PET/MRI, supplied by Mediso USA, Boston and nanoScan SPECT/CT, Mediso, Hungary installed in HCI at the Center for Quantitative Cancer Imaging) to show the strong drug response.
“We were able to observe that the combination of these two drugs — which, when used individually, don’t have much of an impact on the disease — appears to have a very potent impact on the growth of pancreatic cancer,” says McMahon, PhD, a cancer researcher at HCI and Professor of Dermatology. “We have observed this in the lab in petri dishes, then in mouse models, and now in a pancreatic cancer patient on a compassionate use basis. Indeed, we proceeded from a petri dish to a patient in less than two years — a timeline that is rarely seen in medical science.”

Preclinical imaging. Mice were anesthestized and injected by approximately 0.5 mCi of [18 F]-fluorodeoxyglucose (FDG). CT imaging was performed using a NanoScan SPECT/CT scanner followed by PET and MRI imaging using a NanoScan PET/MRI scanner (Mediso Medical Imaging, Budapest). The animal remained anesthetized and immoblized in a common MultiCell animal chamber to provide intrinsic spatial co-registration of CT, MRI, and PET images. T1-weighted Gradient Echo (GRE) images and T2-weighted 2D Fast Spin Echo (FSE) images were acquired prior to initiating a 20-minute PET emission scan at 60 minutes post-injection of FDG. (Figures from this paper are publicly accessible at: https://www.nature.com/articles/s41591-019-0367-9)
At Mediso USA, we are proudly supporting researchers with state-of-the-art advanced imaging techniques in their efforts to shorten time from bench to clinic. Huntsman Cancer Institute Becomes has been the First Mediso Preclinical Imaging Center of Excellence in North America since 2015. They are the first USA site site using the nanoScan 3Tesla PET/MRI.
By accepting you will be accessing a service provided by a third-party external to https://medisousa.com/

