Innovation Delivered
The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule
Maryam Oroujeni1, Tianqi Xu1, Katherine Gagnon2,3, Sara S. Rinne3, Jan Weis4, Javad Garousi1, Ken G. Andersson5, John Löfblom5, Anna Orlova3,6, Vladimir Tolmachev1,6
Iodine‑124 PET quantification of organ‑specific delivery and expression of NIS‑encoding RNA
Matthias Miederer1, Stefanie Pektor1, Isabelle Miederer1, Nicole Bausbacher1, Isabell Sofia Keil2,
Hossam Hefesha3, Heinrich Haas3, Ugur Sahin2,3 and Mustafa Diken2,3
1 Department of Nuclear Medicine, University Medical Center of Johannes Gutenberg University, Mainz, Germany
2 TRON - Translational Oncology at the University Medical Center, Johannes Gutenberg University Mainz GmbH, Mainz, Germany
3 Biopharmaceutical New Technologies (BioNTech) SE, Mainz, Germany
https://doi.org/10.1186/s13550-021-00753-2
Summary
There has been increased interest in the development of mRNA-based vaccines for protection against various infectious diseases and also for cancer immunotherapies since lipid-based nanoparticles opened the possibility to deliver RNA to specific sites within the body, overcoming the limitation of rapid degradation in the bloodstream. In the present study, RNA-lipoplex nanoparticles were assembled by complexing sodium-iodide-symporter (NIS) coding mRNA with liposomes at different charge ratios. Two kinds of RNA-lipoplex systems were used: one system with net anionic charge mediating translation primarily within the spleen, and the other with net positive charge yielding translation primarily within the lungs. After in vitro analysis of the expression kinetics, mice were iv. injected with the mRNA-lipoplexes then 6h later with 124Iodine. Functional NIS protein translation was investigated by PET/MRI imaging. Results revealed rapid increase of 124Iodine uptake in the spleen or lung compared to control-RNA-lipoplexes (containing non-coding RNA) with minimal background in other organs except from thyroid, stomach and salivary gland (where NIS is physiologically expressed). The strong organ selectivity and high target-to-background acquisition of NIS-RNA lipoplexes indicate the feasibility of small animal PET/MRI to quantify organ-specific delivery of RNA.
Results from nanoScan PET/MRI
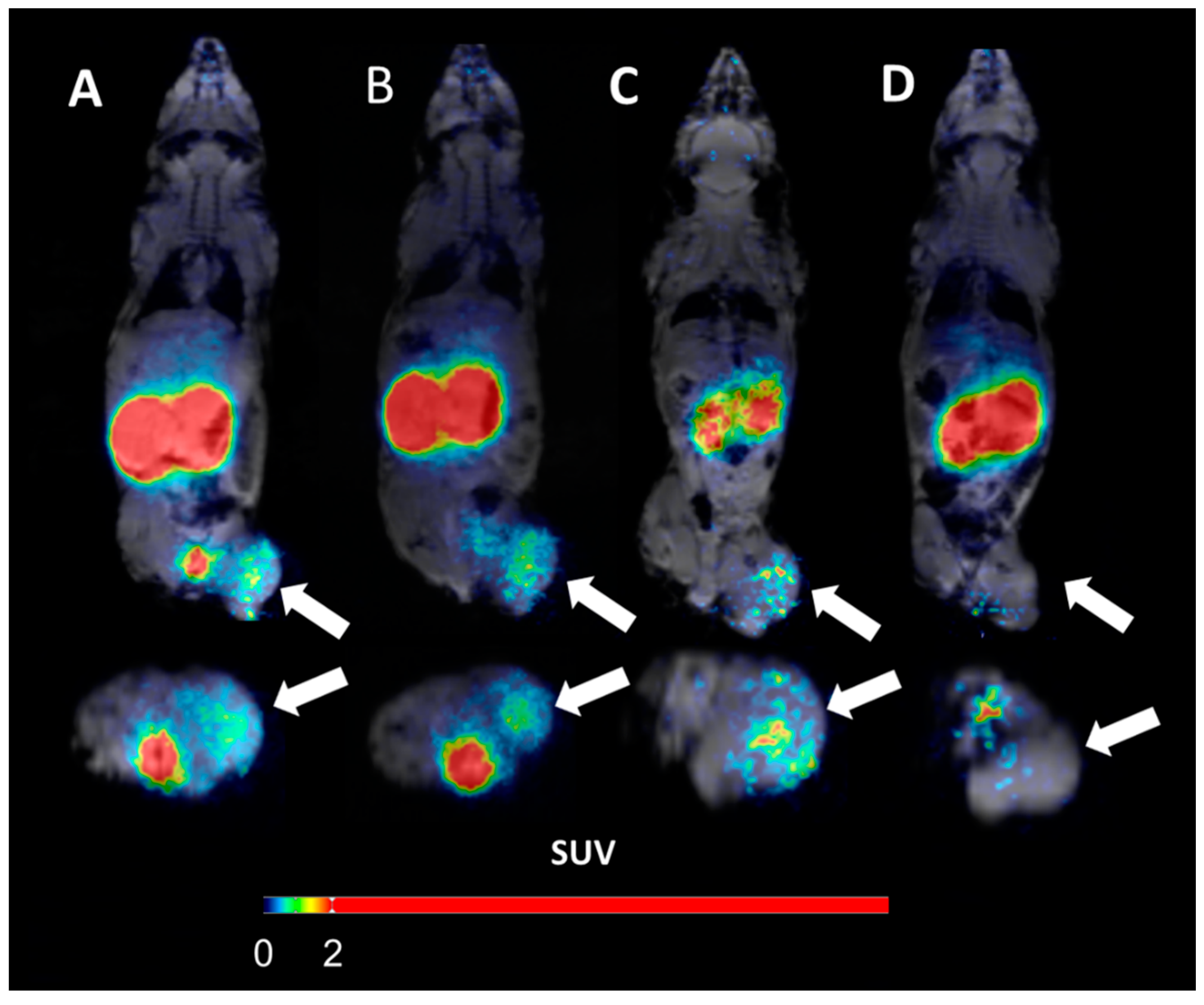
Female BALB/c mice were intravenously injected with RNA-lipoplexes containing 20μg NIS RNA. Six hours later 6.64±0.66MBq 124Iodine was injected intravenously. Three hours after 124Iodine injection, mice were anesthetized and static imaging was performed over 20min by nanoScan PET/MRI. Additionally, one animal per group was imaged dynamically for one hour.
- PET/MRI of anionic NIS-RNA lipoplexes showed a visually detectable increase of 124Iodine uptake in the spleen compared to control-RNA lipoplexes. Due to the high physiological NIS expression in the adjacent gastric wall, this increase was only visually clear with anatomical correlation by MRI. On PET imaging, spleen uptake appeared as an irregularity of the gastric wall which is not detected in control animals
- Lung uptake of NIS-RNA transported by cationic RNA-lipoplexes was depicted more clearly due to larger organ size and no adjacent physiological NIS uptake
- The quantified radioactivity from imaging matched well with the extent of uptake as measured in organs ex vivo, showing enhanced uptake of NIS-RNA and expression of functional NIS-protein in lung or spleen compared to the control RNA
- The uptake in lung was rapid and remained high over the first hour of dynamic acquisition

In vivo imaging with 18F-FDG- and 18F-Florbetaben-PET/MRI detects pathological changes in the brain of the commonly used 5XFAD mouse model of Alzheimer's Disease
18F-FDG-PET Detects Drastic Changes in Brain Metabolism in the Tg4–42 Model of Alzheimer’s Disease
Caroline Bouter1, Philipp Henniges2, Timon N. Franke2, Caroline Irwin2, Carsten Oliver Sahlmann1, Marius E. Sichler2, Nicola Beindorff3, Thomas A. Bayer2 and Yvonne Bouter2
1Department of Nuclear Medicine, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
2Division of Molecular Psychiatry, Department of Psychiatry and Psychotherapy, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
3Berlin Experimental Radionuclide Imaging Center (BERIC), Charité—University Medicine Berlin, Berlin, Germany
https://doi.org/10.3389/fnagi.2018.00425
Summary
The authors have used one of the latest mouse Alzheimer’s Disease (AD) models (Tg4-42 transgenic mutation, which overexpresses the Ab4-42 peptide, which is truncated on the N-terminal region, causing neurotoxicity and Ab aggregation, which are similar to AD). As the AD patients show altered glucose metabolism, the authors chose to follow it with the most common radiopharmaceutical used in PET, namely 18F-FDG, and how it can be used together with the MRI as an early biomarker for AD.
They have found that Tg4-42 mice show a reduction of glucose-metabolism, which correlates with their age, and the decreased 18F-FDG uptake can be shown in early age (3 months).
Results from nanoScan PET/MRI 1T
For the PET/MRI studies, the authors have used a nanoScan PET/MRI 1T, which could provide a fast measurement method together with good statistics. Young (3-4 months) and aged (7-8 months) Tg4-42 (n=7, female) and aged WT C57Bl/6J (7-8 months, n=5, female) control mice were used in this study. The authors have followed the standard 18F-FDG PET/MRI protocol, as the mice were fasted overnight, and 9-21 MBq activity was injected into the tail vein, with a 45 minute long uptake period.
The PET scans were performed for 20 minutes , which was followed by a Tera-Tomo 3D reconstruction method with a 0.3 mm3. For the MRI, the authors have used GRE sequence as a material map for attenuation and scatter correction in the PET reconstruction, and as a brain atlas. The analysis was performed with PMOD software.
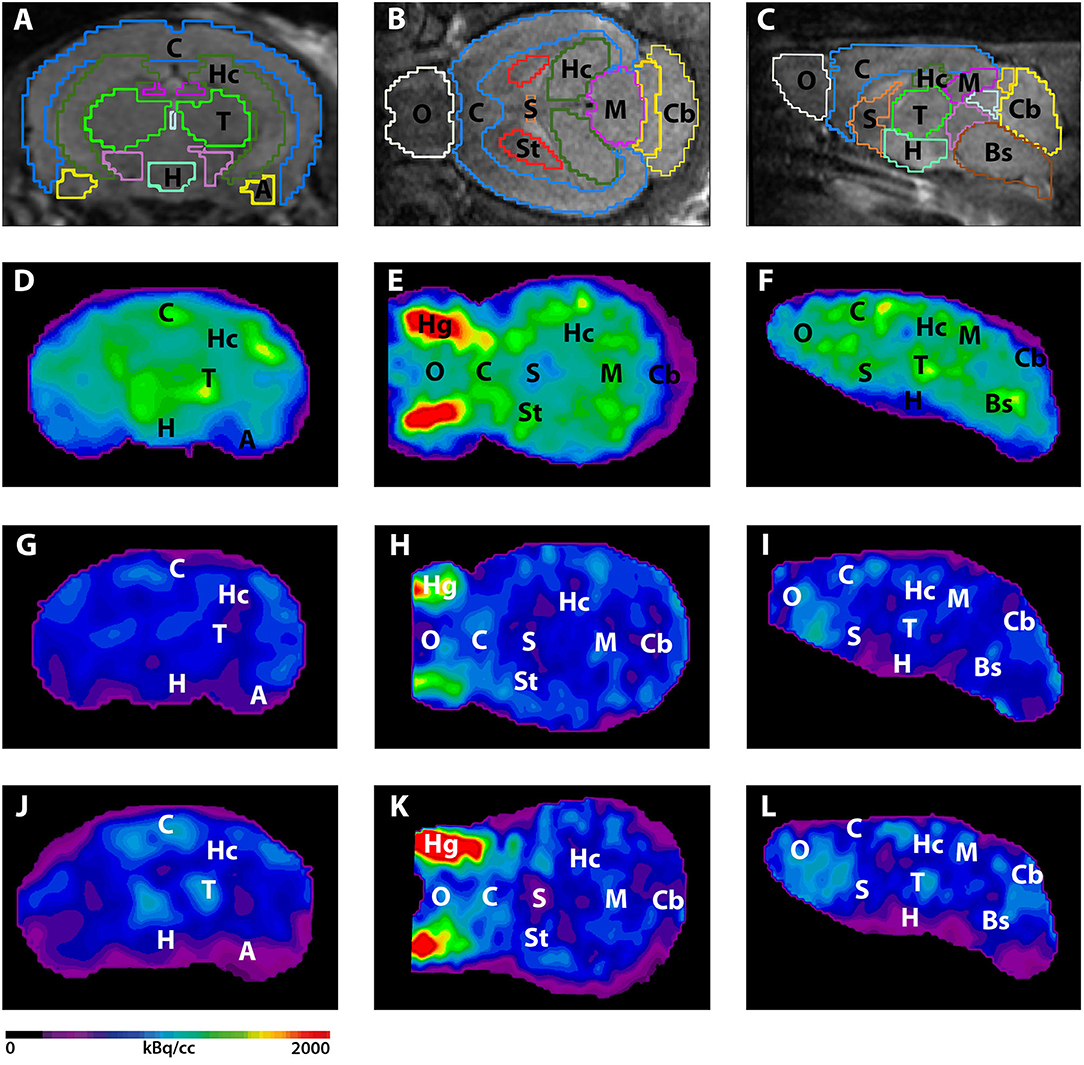
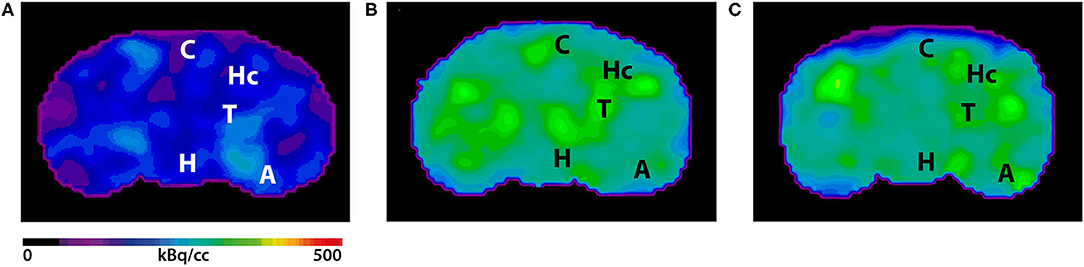
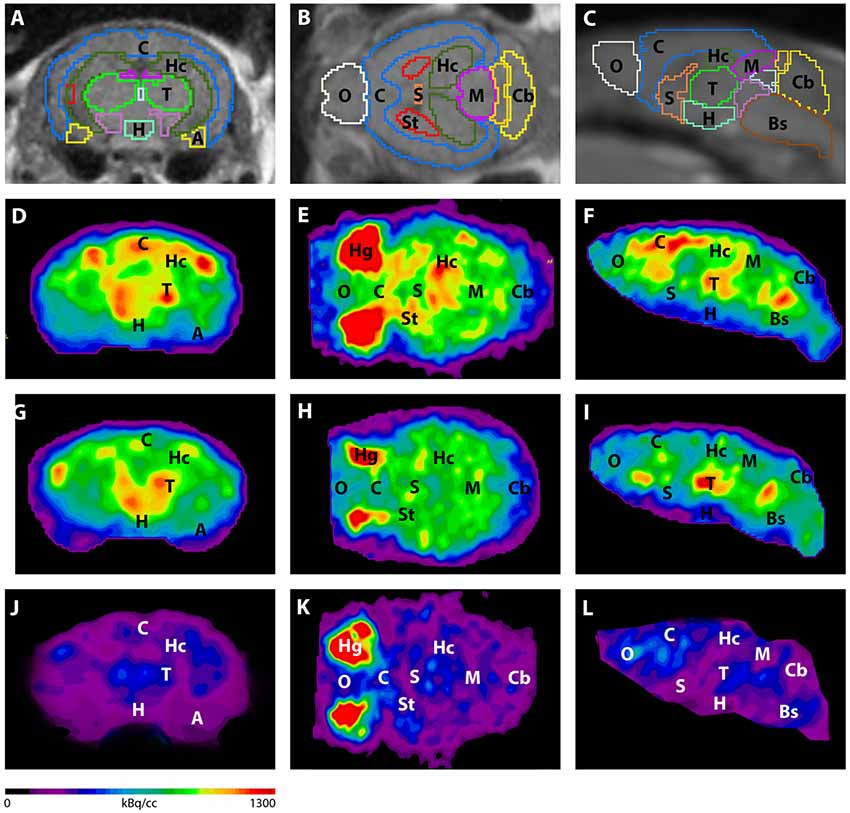
Figure 3. shows the main results from the PET/MRI acquisitions: a-c) MRI images were matched with predefined brain regions; axial, coronal and sagittal view. d-f) 18F-FDG-PET images of a WT mouse. g-i) 18F-FDG-PET images of a young Tg4–42 mouse. j-l) 18F-FDG-PET images of an aged Tg4–42 mouse. A, Amygdala; Bs, Brain Stem; C, Cortex; Cb, Cerebellum; H, Hypothalamus; Hc, Hippocampus; Hg, Harderian glands; M, Midbrain; O, Olfactory Bulb; S, Septum/Basal Forebrain; St, Striatum; T, Thalamus.
- These results suggest that 18F-FDG uptake was distinctly lower in aged Tg4–42 mice compared to WT mice. In young Tg4–42 mice 18F-FDG the uptake did not show significant differences in whole brain uptake but it was reduced in the hippocampus, forebrain, hypothalamus, amygdala and midbrain.
- The study showed that Tg4-42 can be a useful AD model to monitor the effects of various therapeutic strategies in vivo using 18F-FDG uptake as a therapeutic readout.
Cerebrovascular disease encompasses a range of pathologies that affect different components of the cerebral vasculature and brain parenchyma. Large artery atherosclerosis, acute cerebral ischaemia, and intracerebral small vessel disease all demonstrate altered metabolic processes that are key to their pathogenesis. Positron Emission Tomography (PET) can detect and quantify metabolic processes that are relevant to each facet of cerebrovascular disease. The review article published in the November 2017 issue of Nature Reviews Neurology describes how PET-based imaging of metabolic processes at the neurovascular interface has contributed to our understanding of cerebrovascular disease.
Evans, N. R. et al. PET imaging of the neurovascular interface in cerebrovascular disease. Nat Rev Neurol 13, 676–688 (2017). doi:10.1038/nrneurol.2017.129
PET imaging employs various radioligands to detect physiological processes in vivo. The article written by Nicholas R. Evans, University of Cambridge, Cambridge, UK and his colleagues summaries the radioisotopes of PET ligands used for the following list of cellular or physiological targets of vascular biology, actute ischaemic stroke and small vessel disease:
- Increased metabolic rate (inflammation): 18F-FDG
2. Macrophages: 68Ga-DOTATATE (targeting somatostatin receptor type 2)
3. Microcalcification: 18F-NaF (hydroapatite)
4. Hypoxia: 18F-FMISO (targeting selective reduction in hypoxia)
5. Macrophages and microglia: 11C-PK11195, 11C-PBR28, 18F-DPA-714, 11C-vinpocetine, 18F-GE-180 (all targeting TSPO)
6. Neurons: 11C-FMZ (GABA-A receptor)
7. Amyloid: 11C-PiB (analogue of thioflavin T)
8. Neurons: 18F-FNDP (epoxide hydrolase enzyme)
9. Expressed on neurons, astrocytes, microglia and endothelial cells: 18F-NS14490 (α7 nicotonic acetylcholine receptor)
10. Apoptosis: 18F-labeled isatins (caspase 3, caspase 7)
The review article considers sensitivity, specificity, technical considerations and also clinical implications for each radiotracersThe nanoScan PET/MRI3T is an ideal combination of modalities for research of cerebrovascular diseases: structural imaging provided by MRI is co-registered and combined with the PET ability to detect and quantify these pathophysiological processes in vivo. Information obtained from PET studies has helped to shape the understanding of key concepts in cerebrovascular medicine, including vulnerable atherosclerotic plaque, salvageable ischaemic penumbra, neuroinflammation and selective neuronal loss after ischaemic insult. New PET ligands continue to be developed that have superior specificity or that target new processes of interest.
In the first published article from MSKCC (Carney, B. et al. Non-invasive PET Imaging of PARP1 Expression in Glioblastoma Models. Mol Imaging Biol 1–7 (2015)), using the nanoScan PET/MRI (1T) small animal imaging system, in vivo whole body PET/MRI imaging of [18F]PARPi in orthotopic brain tumor-bearing mice is referenced.
[18F]PARPi is a selective PARP1 imaging agent that can be used to visualize glioblastoma in xenograft and orthotopic mouse models with high precision and good signal/noise ratios offering new opportunities to non-invasively image tumor growth and monitor interventions.
Figure 6 in the article shows coronal views of contrast-enhanced MRI, [18F]PARPi PET images, and fused PET/MRI of orthotopic U251 MG tumor-bearing mice. In the top row the mouse receivied only [18F]PARPi, in the bottom row the mouse receivied [18F]PARPi after a 500-fold excess of olaparib.

The animals were injected with 200 µCi of [18F]-PARPi and a 20 minutes static PET scan was acquired 2 hours post injection. 200 µL of diluted gadopentate dimegumine in saline solution was administered intravenously one minute prior to MRI acquisition. Tumor regions were identified on anatomic images acquired using a post-contrast T-weighted spin-echo (SE) acquisition. The co-localization of [18F]PARPi and tumor in PET/MRI studies was confirmed by ex vivo autoradiography. In PET/MRI fusion images, accumulation in the tumor was co-aligned with the orthotopic tumor on MRI. In mice receiving an injection of olaparib ahead of the radiotracer, the [18F]PARPi tumor uptake was negligible.
It's important to note that no further or manual co-registration was required at all as the PET/MRI studies performed on the nanoSCan PET/MRI are co-registered by nature due to the common gantry and automated acquisition system. The very same images are displayed in the viewer when the dual-modality study is loaded from the DICOM server after reconstruction. This gives scientists confidence when evaluating multi-modal data; changing animal physiology and data obtained at different times won't distort the findings.
Development of dual-modality, aluminium hydroxide stabilised magnetic nanoparticles probes is published in the Biomaterials 2014 July issue. The main author of the article titled ‘Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging’ [1] is Dr. Xianjin Cui, member of Philip Blower’s group at King's College London, Division of Imaging Sciences and Biomedical Engineering. The article is a collaboration between researchers from King’s College London (UK), Nottingham University (UK), Aston University (UK), CROmed Ltd. (Hungary). This is an open access article. Download the Article in PDF, Appendix A in Word.
Superparamagnetic nanoparticles
Superparamagnetic nanoparticles (NPs) have been intensively investigated due to their potential applications in biosensors, targeted drug develivery, MRI and localised hyperthermia induction. The problem with these nanoparticles is that they tend to aggregate to minimize the surface energy. Bio-applications require colloidal stability and dispersibility in water and biological environments. There are several methods described in the literature to obtain stable colloids of magnetic nanoparticles. A simple approach is presented in the article to stabilise magnetic nanoparticles by coating them with an Al(OH)3 layer via a hydrolysis process for conjugation. The use of an inorganic shell material introduces stability, functionality (nanoparticle recognised by the macrophage-monocytic system) and water-solubility. The materials, general characterisation, synthesis and radiolabelling are described in the article.
in vivo PET/MR imaging
What is interesting for our blog is that for in vivo PET/MR imaging of the agents on mice were performed on the integrated nanoScan preclinical PET/MRI imaging system installed at the Nanobiotechnology & In Vivo Imaging Center, Semmelweis University in Budapest, Hungary.
The total injected F-18 activity was 0.95 MBq (25.7 microCi). PET scanning was started immediately after injection and continued for 120 min. Acquisition took place in 1–5 coincidence mode with 5 ns coincidence window, 400–600 keV energy window. MR scanning was performed immediately after PET. A 3D expectation maximisation (3D EM) PET reconstruction algorithm (Mediso Tera-Tomo™) was applied to produce PET images including corrections for attenuation and scatter, dead time, decay and randoms. After 8 iterations the reconstruction stopped resulting in images with 0.1 mm voxel size and time frames of 8 × 15 min. The images of the two modalities were fused automatically.
The PET/MRI fused image is presented in the Appendix A. of the article. The injected activity was only 0.95 MBq (25.7 microCi) and the PET images show only 15 minutes of acquisition!
 In vivo PET/MRI images of a normal young C57BL/6 mouse using 18F radiolabelled 3: (a) whole body PET image showing distribution of 18F 30 minutes post injection (maximum intensity projection, mice in prone position); (b) PET/MRI fused image (coronal section, 0-15 minutes); (c) PET/MRI fused image (coronal section, 105-120 minutes); (d) MR image prior to the injection of NPs, and (e) MR image post the injection of NPs, showing a darkening contrast at lung and live area. Due to the unstable Al(OH)3 shell, 18F-fluoride radioactivity was released from magnetic NPs 3 within 15 minutes and localised in bone.
In vivo PET/MRI images of a normal young C57BL/6 mouse using 18F radiolabelled 3: (a) whole body PET image showing distribution of 18F 30 minutes post injection (maximum intensity projection, mice in prone position); (b) PET/MRI fused image (coronal section, 0-15 minutes); (c) PET/MRI fused image (coronal section, 105-120 minutes); (d) MR image prior to the injection of NPs, and (e) MR image post the injection of NPs, showing a darkening contrast at lung and live area. Due to the unstable Al(OH)3 shell, 18F-fluoride radioactivity was released from magnetic NPs 3 within 15 minutes and localised in bone.
The reconstruction features the TeraTomo algorithm's latest version which will be available for all our sites this autumn. In our opinion it is hard to get better bone images nowadays with PET for such a low injected activity than it’s featured in this article. Funnily enough noone intended to make bone images as this is a proof that the radiolabel went off from the nanoparticles and trapped in bones of the mouse. Remember, this is not a F-18 flouride bone scan! The ‘grainy’ PET image isn't the result of any regularization issue – this represents the real uneven flour uptake in the bones. You can notice the anatomical features of the knee joint – the patella, condyles of femur can be distinguished as well!
Read more about the integrated, automated small animal whole-body PET/MRI system.
[1] Cui, X. et al. Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging. Biomaterials doi:10.1016/j.biomaterials.2014.04.004
 This article was published in discovered, The HZDR Research Magazine (Issue 02.2013, December 2013/January 2014, ISSN: 2194-5713; PDF 2.2MB)
This article was published in discovered, The HZDR Research Magazine (Issue 02.2013, December 2013/January 2014, ISSN: 2194-5713; PDF 2.2MB)
Six white CD-1 mice are scurrying through the litter in their cage, climbing the metal bars, nibbling away at the pellets they are being fed, and snuggling with each other. What they don't yet know is they're about to participate in a pivotal study. One that will save lives - those of mice and, one day, of men. As part of his dissertation, Mathias Kranz, Ph.D. student at the HZDR Research Site Leipzig, is currently investigating the degree of radioactivity that builds up within the bodies of mice whenever radioactive probes - called radiotracers - are used, and identifying in which organs specifically it accumulates. Eventually, these data will be extrapolated to the human magnitude. Radiotracers are chemical compounds that include a radioactive element of some sort, which can help scientists observe metabolic processes in living organisms.
Specifically, in the case of the Leipzig project, we're talking about the two radiotracers [18F]fluspidine and [18F]flubatine - both of them molecules containing the radionuclide 18F (fluorine). They're supposed to ultimately find their way into the diagnostics of cancers and neurodegenerative diseases like Alzheimer's. Key is their ability to imitate properties of various endogenous structures.
Before a radioactive probe is ready for use in the hospital setting, its efficacy and safety must first be documented in living organisms.
Once injected into the human body, they bind with high affinity to certain targets - in the case of the "PET sugar" [18F]FDG, which is also used at the Leipzig site, highly metabolically active tissues like tumors. The emitted radiation from the radioactive molecules can be captured and subsequently analyzed using positron emission tomography (PET). However, before a radioactive tracer can be introduced into the hospital setting, its efficacy and safety to the living organism must first be confirmed. This is a prerequisite imposed by the German Federal Office for Radiation Protection (BfS) and the Federal Institute for Drugs and Medical Devices (BfarM). This multistep procedure starts with work on mice and occasionally pigs and ultimately leads to research conducted on healthy human subjects. Here, the HZDR scientists are receiving support from their colleagues at the Clinic for Nuclear Medicine at Leipzig University Hospital.
Leipzig as reference site
As of spring 2013, when operations by experienced colleagues at the HZDR main site Dresden first commenced, Germany's first-ever commercial full-body PET/MRI for small animals opened in Leipzig - one of only a few worldwide. The HZDR is the reference site for Hungarian manufacturer Mediso (Budapest) - which brings with it a number of obvious benefits: "There are still a handful of delayed-onset childhood illnesses but whenever we do report any problem, help typically arrives within a matter of hours," Mathias Kranz explains. The 27-year-old fellow, who holds a master's in engineering, studied biomedical technology at Ilmenau University of Technology, and has been working at the HZDR Institute of Radiopharmaceutical Cancer Research for about a year now. He is thrilled with the new device: "Not only does it allow us to obtain information about metabolic processes that are happening inside the body, it also yields high-resolution three-dimensional images that document the exact location and distribution of soft tissues." especially when it comes to brain imaging, MR devices yield far better results than conventional PET and computer tomography (CT) combinations.
The mice remain safe
"Without these methods, we would need to dissect the animal subjects, remove individual organs, and then measure them in order to determine the degree of radioactivity that has accumulated in the body following injection of the radiotracer. What's interesting is not only the current dose rate but also how it changes over the course of minutes and hours, which helps determine the organ dose. Thanks to PET/MRI, we're able to conduct even long-term studies using the same exact mouse," Mathias Kranz explains. In the case of other methods, one laboratory animal has to be sacrificed each time a single measurement is obtained.
During examination, the mice are lying on a heated animal bed, their breathing monitored with the help of a pressure sensor. The radioactively labeled substance is injected into the tail vein. The mice are fully anesthetized and won't remember anything afterwards. On a screen, Mathias Kranz is now examining a black and grey image showing the inside of the mouse's body. Red, yellow, and blue spots are lighting up in certain body regions. "Red means these are sites where there is a high degree of radioactivity, in other words that a lot of our substance was deposited in these places," the young scientist explains. At first glance, the liver, kidneys, and bladder are easily recognized - organs, which are actively involved in the substance's elimination from the body.
After the experiments are done, Mathias Kranz calculates the expected effective human dose. This serves as a risk-assessment at the time of introducing the probes into the clinical setting. Based on their results, the researchers have filed for approval of a study with the BfS for use of their newly developed radiotracers (+)-[18F]flubatine and (S)-(-)-[18F]fluspidine in humans. The scientists are working closely with their colleagues at Leipzig University Hospital, Department of Nuclear Medicine, on these projects. The projected start date is early 2014.
Hyperpolarized 13C imaging approach increases the MR signal more than 20,000 times for studying real-time metabolism of disease. Metabolic MRI with hyperpolarized agents shows promise by helping support the differentiation of benign and malignant lesions, separating aggressive from slow-growth tumors and facilitating non-invasive treatments.
The Need for Speed
Molecular Imaging describes techniques that directly or indirectly visualize, characterize, and measure the distribution of molecular or cellular processes at the molecular and cellular levels in humans and other living systems.
The most suitable modalities for small-animal in vivo imaging applications are based on nuclear medicine techniques (essentially, positron emission tomography [PET] and single photon emission computed tomography [SPECT]), optical imaging (OI), computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy imaging (MRSI), and ultrasound.
Conventional magnetic resonance imaging (MRI) relies on magnetic resonance (MR) signal from proton nuclei of water within the body. The MR signal is encoded with magnetic field gradients for 2D and 3D imaging with no fundamental barriers to spatial resolution as long as sufficient MR signal is available.MRI provides excellent contrast and spatial resolution without radiation exposure - however one limitation of MRI in particular is low sensitivity, especially when compared to PET or SPECT.
Hyperpolarization
Hyperpolarization may address this problem by polarizing spins of a nucleus by several orders of magnitude that seen at thermodynamic equilibrium. However this technique practically doesn't work in water, because spins return back to their equilibrium state, i.e. very low polarization, within seconds. 3He, 13C, 15N, 129Xe and other nuclear spins can be hyperpolarized to the order of near unity resulting in signal enhancement by 4-6 orders of magnitude. Moreover, the decay of their hyperpolarized spin state can be as long as several hours - making useful chemical compounds as hyperpolarized contrast agents. These agents are prepared by physical and/or chemical manipulations followed by administration of these contrast agents in living organisms and their MRI or MRSI imaging.
Hyperpolarized (HP) 129Xe and 3He have been achieved by optical pumping, with potential for low-radiation imaging of the lungs. For nuclei found in endogenous molecules (in particular carbon and nitrogen), the dynamic nuclear polarization (DNP) technique has emerged as a way to polarize small-molecule metabolites. Briefly, 13C-labeled molecules, doped with small quantities of a stable radical, are cooled to approximately 1 K in a magnetic field; microwave irradiation transfers polarization from the fully polarized electron spins on the radical to the 13C nuclei. The sample is then rapidly dissolved using a hot pressurized solution, which can be injected into an animal (or human) in a separate imaging magnet.
Opening the fourth dimension by Chemical Shift Imaging
This approach increases the MR signal more than 20,000 times, thus increasing the biological sensitivity of hyperpolarized MR imaging. Hyperpolarized contrast agents are similar to radioactive tracers in that their signal- generating capability decays exponentially with time - similar to SPECT and PET tracers. The dramatic signal enhancements obtained allow not only the detection of the introduced metabolic agent, but also its metabolic products in real-time. This enabled by magnetic resonance spectroscopic imaging (MRSI) offering the fourth dimension of chemical shift reporting on composition of tissue, i.e. imaging of protons of metabolites in tumors, cardiac tissue and brain, in addition to three spatial dimensions. Its biggest application so far has been in imaging the glucose consumption in tumors — glucose and lactate for the localization of benign and malignant prostate cancer. this concept has a lot of potential for other kinds of metabolic applications, too, most notably diabetes imaging.
Despite signal boost by several orders of magnitude, hyperpolarized MRI relies on signal from relatively dilute spins of administered hyperpolarized contrast agents. For example, hyperpolarized 13C-lactate concentration in vivo is on the order of a few mM, which is several orders of magnitude lower than proton concentration of tissue water. As a result, SPECT and PET are inherently significantly more sensitive (by orders of magnitude) imaging modalities when accounting for contrast agent quantity. When comparing hyperpolarized MRI to PET imaging, it should also be noted that the vast majority of hyperpolarized contrast agents have significantly shorter lifetime on the order, of 0.5-5 minutes in vivo. This double-edged sword limits the use of hyperpolarized contrast agents from the perspective of metabolic pathways penetration, contrast agent in vivo delivery, pharmaceutical preparation and imaging site distribution. On the other hand, it offers an opportunity to perform a repeat scan within minutes after initial hyperpolarized scan, because there is no background signal from the first initially administered dose.
Bringing it into one system
PET/MR imaging is just a phenomenal tool — it combines two very strong technologies. This field however opens even more new opportunities by potentially combining the power of molecular imaging of hyperpolarized MRI and high sensitivity PET. While the main advantage of hyperpolarized MRI is the large sensitivity boost enabled by increased nuclear spin polarization, this increase is not endowed by the magnetic field of the MRI scanner. As a result, it is possible to perform MRI of hyperpolarized contrast agents in very low magnetic fields. The nanoScan PET/MRI is equipped with a permanent 1T magnet which is seamlessly integrated and automated into the equipment. Our advantage is the inherently low cost maintenance, because the need for a high-field cryogenic magnet is eliminated and also no other site preparation and supportive maintenance, like water cooling is required. The combination of low cost and sub-second scan speed is a clear advantage.
Further readings
The hyperpolarized MRI is and emerging and quickly developing field, however its importance can assessed by the increasing number of published articles and presentations on conferences. Recently a review article was published on 13C hyperpolarized magnetic resonance using dynamic nuclear polarization in Chemical Society Reviews written by Kayvan R. Keshari and David M. Wilson.
Suggested literature
- Keshari, Kayvan R., and David M. Wilson. "Chemistry and Biochemistry of 13C Hyperpolarized Magnetic Resonance Using Dynamic Nuclear Polarization." Chemical Society Reviews 43, no. 5 (February 10, 2014): 1627–59. doi:10.1039/C3CS60124B.
- Gallagher, Ferdia A., Sarah E. Bohndiek, Mikko I. Kettunen, David Y. Lewis, Dmitry Soloviev, and Kevin M. Brindle. "Hyperpolarized 13C MRI and PET: In Vivo Tumor Biochemistry." Journal of Nuclear Medicine 52, no. 9 (September 1, 2011): 1333–36. doi:10.2967/jnumed.110.085258.
- Chekmenev, Eduard Y. MRI "Hyperpolarization and Molecular Imaging" mi Gateway, Newsletter of the SNMMI CMIIT, Vol. 7, Issue 3, 2013-3
The suggested reading list was actually used to prepare this post. This was an introductory post in the realm of HP MRI imaging - hope you enjoyed it.
Last month, in October a new review article titled Preclinical Imaging: an Essential Ally in Modern Biosciences on preclinical imaging technologies was published in the Molecular Diagnosis & Therapy journal. The journal provides insights into the latest molecular diagnostic and pharmacogenomic techniques and their use in personalized medicine.
Cunha, Lídia, Ildiko Horvath, Sara Ferreira, Joana Lemos, Pedro Costa, Domingos Vieira, Dániel S. Veres, et al. 2013. “Preclinical Imaging: An Essential Ally in Modern Biosciences.” Molecular Diagnosis & Therapy: 1–21. doi:10.1007/s40291-013-0062-3.
The find out that actually what is small-animal or preclinical imaging, P. Zanzonico from MSKCC has provided a good definition, stating that 'it constitutes a way of assessing biological structures and function in vivo by noninvasive means, allowing the collection of quantitative information, both in health and disease states' [1].
The main role of preclinical imaging is to deliver translational answers for serious health-related problems of the growing and aging world population. Small animal models have to represent a bridge between discoveries at the molecular level and clinical implementation in diagnostics or therapeutics. Small animal imaging is being used in a wide variety of lines of research, especially in infection, inflammation, oncology, cardiology, and neurosciences.
The article summarizes the general properties of diagnostic imaging modalities and reviews them one-by-one including Positron emission tomography (PET), Single photon emission computed tomography (SPECT), Optical imaging (OI), Computed tomography (CT), Magnetic resonance imaging (MRI) and Ultrasound (US) and their related instrumentation of these modalities in small animal imaging. A separate and well detailed section is dedicated to the comparison of micro-SPECT and micro-PET. The general parameters are summarized in a large table listing imaging characteristics (spatial resolution, sensitivity, penetration depth, temporal resolution), related costs, probe types, major advantages, disadvantages and their application areas.
There are inherent limitations to each imaging modality - this has brought commercial multi-modality systems 10+ years ago to the market. Multimodal combination has enabled some of the most important limitations of each imaging modality to be overcome when used alone. The considerations are explained in the tenth sections of the article.
It's an honor to see multi-modality images of PET/MRI and SPECT/MRI acquired by our nanoScan imagers in the article.
A SPECT/MRI application was selected as the image of this blog post. The image shows transverse slices of SPECT and MRI images of a mouse brain. SPECT was acquired using a specific agent for cortical benzodiazepine receptors (123I-NNC13-82431). The lack of anatomical information of SPECT acquisition is complemented with the information provided by MRI, in which the eyes, the olfactory bulbs and the first and second ventricles are shown. The multimodality SPECT/MRI image provides information about functional benzodiazepine receptors from SPECT allied to good soft tissue contrast from the MRI.
Abstract of the Article
Translational research is changing the practice of modern medicine and the way in which health problems are approached and solved. The use of small-animal models in basic and preclinical sciences is a major keystone for these kinds of research and development strategies, representing a bridge between discoveries at the molecular level and clinical implementation in diagnostics and/or therapeutics. The development of high-resolution in vivo imaging technologies provides a unique opportunity for studying disease in real time, in a quantitative way, at the molecular level, along with the ability to repeatedly and non-invasively monitor disease progression or response to treatment. The greatest advantages of preclinical imaging techniques include the reduction of biological variability and the opportunity to acquire, in continuity, an impressive amount of unique information (without interfering with the biological process under study) in distinct forms, repeated or modulated as needed, along with the substantial reduction in the number of animals required for a particular study, fully complying with 3R (Replacement, Reduction and Refinement) policies. The most suitable modalities for small-animal in vivo imaging applications are based on nuclear medicine techniques (essentially, positron emission tomography [PET] and single photon emission computed tomography [SPECT]), optical imaging (OI), computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy imaging (MRSI), and ultrasound. Each modality has intrinsic advantages and limitations. More recently, aiming to overcome the inherent limitations of each imaging modality, multimodality devices designed to provide complementary information upon the pathophysiological process under study have gained popularity. The combination of high-resolution modalities, like micro-CT or micro-MRI, with highly sensitive techniques providing functional information, such as micro-PET or micro-SPECT, will continue to broaden the horizons of research in such key areas as infection, oncology, cardiology, and neurology, contributing not only to the understanding of the underlying mechanisms of disease, but also providing efficient and unique tools for evaluating new chemical entities and candidate drugs. The added value of small-animal imaging techniques has driven their increasing use by pharmaceutical companies, contract research organizations, and research institutions.
[1] Zanzonico P. Noninvasive imaging for supporting basic research. In: Kiessling F, Pichler BJ, editors. Small animal imaging—basics and practical guide. Heidelberg: Springer; 2011. p. 3–16. (Springer; Google Books)
Back in May 2013, I gave a talk titled "The Motivations and Systems for High Content In-vivo Tomographic Imaging in
Drug Discovery" at the 6th Imaging in Drug Discovery & Development Conference in Boston. Mediso USA was the Silver Sponsor for the event.
According to GTC this is "the only imaging conference that brings together high-level/influential leaders with decision-making authority from the pharmaceutical industry, academia, and government to share their knowledge and expertise in drug discovery and development". Needles to say, the sessions were indeed interesting, with an interesting mix from academia, government, pharma and imaging companies.
Session topics included:
- Advantages and Challenges of Available Imaging Modalities
- Translational Imaging Applications: Preclinical to Clinical
- Imaging Applications Across Multiple Therapeutic Areas
- Molecular Imaging and Diagnostic Approaches and Capabilities
- High Content Imaging, Quantitative Imaging and Modeling Capabilities
The November issue of the was published today in the Genetic Engineering & Biotechnology News, with the Feature Article: Raising the Bar in Preclinical Imaging written by MaryAnn Labant. The article is based on presentations given at the May GTC Imaging in Drug Discovery and Development Conference.
Please find below our related section from the second page of the online article.
Integrated Imaging Systems
Preclinical PET scanners with an integrated microCT have substantially improved the anatomical registration of PET predominately to the skeleton, yet little progress has been made in soft tissue contrast, even with the use of a CT contrast agent.
 Integrated PET/MRI or SPECT/MRI systems offer many benefits. MRI uses no radiation, offers better soft tissue contrast, and provides molecular readouts. To date, preclinical PET imaging combined with MRI has been performed using two independent systems and a bespoke co-registration algorithm to fuse the images.
Integrated PET/MRI or SPECT/MRI systems offer many benefits. MRI uses no radiation, offers better soft tissue contrast, and provides molecular readouts. To date, preclinical PET imaging combined with MRI has been performed using two independent systems and a bespoke co-registration algorithm to fuse the images.
Mediso recently commercialized the first serially produced, fully integrated, automated PET/MRI system, the nanoScan PET/MRI, and a fully integrated, automated SPECT/MRI system, the nanoScan SPECT/MRI. Single systems enable use of the same imaging technology, imaging protocol, and biomarkers for small to large subjects.
According to Illes J. Muller, managing partner, preclinical PET/MRI and SPECT/MRI allow combination of radionuclide biomarkers with an MRI contrast agent on a routine basis, an attractive prospect for evaluating new drugs for oncology, neurology, and cardiovascular disease. Now, physiological/metabolic readouts can be combined with high-resolution, soft-tissue contrast as well as dynamic functional perfusion imaging.
 In addition, SPECT provides the ability to perform multi-isotope imaging, probing two or more molecular pathways simultaneously by detecting isotopes with different emission energies, and has no physical limits in resolution. SPECT/MRI technology is less expensive. The labeling is easier, and no on-site cyclotron is required.
In addition, SPECT provides the ability to perform multi-isotope imaging, probing two or more molecular pathways simultaneously by detecting isotopes with different emission energies, and has no physical limits in resolution. SPECT/MRI technology is less expensive. The labeling is easier, and no on-site cyclotron is required.
A potential major application for multimodal emission tomography combined with MRI is quantitative 3D imaging of tumor heterogeneity. To assess the spatial distribution of a given PET or SPECT biomarker within a tumor requires ultra-high resolution and high sensitivity and corrections for tumor perfusion. MRI is able to differentiate between healthy and dead tumor tissue for tumor response evaluation.
Note: This was the related section from the article, with links added to the relevant pages of Mediso USA website.
Blog Image
The blog image shows a Mouse Tumor Heterogeneity Study performed with nanoScan PET/MRI. The mouse was injected with 3 MBq Ga68-DOTA-TATE and imaged for 15 minutes at 60-75 min post injection.