Innovation Delivered
Hyperpolarized 13C imaging approach increases the MR signal more than 20,000 times for studying real-time metabolism of disease. Metabolic MRI with hyperpolarized agents shows promise by helping support the differentiation of benign and malignant lesions, separating aggressive from slow-growth tumors and facilitating non-invasive treatments.
The Need for Speed
Molecular Imaging describes techniques that directly or indirectly visualize, characterize, and measure the distribution of molecular or cellular processes at the molecular and cellular levels in humans and other living systems.
The most suitable modalities for small-animal in vivo imaging applications are based on nuclear medicine techniques (essentially, positron emission tomography [PET] and single photon emission computed tomography [SPECT]), optical imaging (OI), computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy imaging (MRSI), and ultrasound.
Conventional magnetic resonance imaging (MRI) relies on magnetic resonance (MR) signal from proton nuclei of water within the body. The MR signal is encoded with magnetic field gradients for 2D and 3D imaging with no fundamental barriers to spatial resolution as long as sufficient MR signal is available.MRI provides excellent contrast and spatial resolution without radiation exposure - however one limitation of MRI in particular is low sensitivity, especially when compared to PET or SPECT.
Hyperpolarization
Hyperpolarization may address this problem by polarizing spins of a nucleus by several orders of magnitude that seen at thermodynamic equilibrium. However this technique practically doesn't work in water, because spins return back to their equilibrium state, i.e. very low polarization, within seconds. 3He, 13C, 15N, 129Xe and other nuclear spins can be hyperpolarized to the order of near unity resulting in signal enhancement by 4-6 orders of magnitude. Moreover, the decay of their hyperpolarized spin state can be as long as several hours - making useful chemical compounds as hyperpolarized contrast agents. These agents are prepared by physical and/or chemical manipulations followed by administration of these contrast agents in living organisms and their MRI or MRSI imaging.
Hyperpolarized (HP) 129Xe and 3He have been achieved by optical pumping, with potential for low-radiation imaging of the lungs. For nuclei found in endogenous molecules (in particular carbon and nitrogen), the dynamic nuclear polarization (DNP) technique has emerged as a way to polarize small-molecule metabolites. Briefly, 13C-labeled molecules, doped with small quantities of a stable radical, are cooled to approximately 1 K in a magnetic field; microwave irradiation transfers polarization from the fully polarized electron spins on the radical to the 13C nuclei. The sample is then rapidly dissolved using a hot pressurized solution, which can be injected into an animal (or human) in a separate imaging magnet.
Opening the fourth dimension by Chemical Shift Imaging
This approach increases the MR signal more than 20,000 times, thus increasing the biological sensitivity of hyperpolarized MR imaging. Hyperpolarized contrast agents are similar to radioactive tracers in that their signal- generating capability decays exponentially with time - similar to SPECT and PET tracers. The dramatic signal enhancements obtained allow not only the detection of the introduced metabolic agent, but also its metabolic products in real-time. This enabled by magnetic resonance spectroscopic imaging (MRSI) offering the fourth dimension of chemical shift reporting on composition of tissue, i.e. imaging of protons of metabolites in tumors, cardiac tissue and brain, in addition to three spatial dimensions. Its biggest application so far has been in imaging the glucose consumption in tumors — glucose and lactate for the localization of benign and malignant prostate cancer. this concept has a lot of potential for other kinds of metabolic applications, too, most notably diabetes imaging.
Despite signal boost by several orders of magnitude, hyperpolarized MRI relies on signal from relatively dilute spins of administered hyperpolarized contrast agents. For example, hyperpolarized 13C-lactate concentration in vivo is on the order of a few mM, which is several orders of magnitude lower than proton concentration of tissue water. As a result, SPECT and PET are inherently significantly more sensitive (by orders of magnitude) imaging modalities when accounting for contrast agent quantity. When comparing hyperpolarized MRI to PET imaging, it should also be noted that the vast majority of hyperpolarized contrast agents have significantly shorter lifetime on the order, of 0.5-5 minutes in vivo. This double-edged sword limits the use of hyperpolarized contrast agents from the perspective of metabolic pathways penetration, contrast agent in vivo delivery, pharmaceutical preparation and imaging site distribution. On the other hand, it offers an opportunity to perform a repeat scan within minutes after initial hyperpolarized scan, because there is no background signal from the first initially administered dose.
Bringing it into one system
PET/MR imaging is just a phenomenal tool — it combines two very strong technologies. This field however opens even more new opportunities by potentially combining the power of molecular imaging of hyperpolarized MRI and high sensitivity PET. While the main advantage of hyperpolarized MRI is the large sensitivity boost enabled by increased nuclear spin polarization, this increase is not endowed by the magnetic field of the MRI scanner. As a result, it is possible to perform MRI of hyperpolarized contrast agents in very low magnetic fields. The nanoScan PET/MRI is equipped with a permanent 1T magnet which is seamlessly integrated and automated into the equipment. Our advantage is the inherently low cost maintenance, because the need for a high-field cryogenic magnet is eliminated and also no other site preparation and supportive maintenance, like water cooling is required. The combination of low cost and sub-second scan speed is a clear advantage.
Further readings
The hyperpolarized MRI is and emerging and quickly developing field, however its importance can assessed by the increasing number of published articles and presentations on conferences. Recently a review article was published on 13C hyperpolarized magnetic resonance using dynamic nuclear polarization in Chemical Society Reviews written by Kayvan R. Keshari and David M. Wilson.
Suggested literature
- Keshari, Kayvan R., and David M. Wilson. "Chemistry and Biochemistry of 13C Hyperpolarized Magnetic Resonance Using Dynamic Nuclear Polarization." Chemical Society Reviews 43, no. 5 (February 10, 2014): 1627–59. doi:10.1039/C3CS60124B.
- Gallagher, Ferdia A., Sarah E. Bohndiek, Mikko I. Kettunen, David Y. Lewis, Dmitry Soloviev, and Kevin M. Brindle. "Hyperpolarized 13C MRI and PET: In Vivo Tumor Biochemistry." Journal of Nuclear Medicine 52, no. 9 (September 1, 2011): 1333–36. doi:10.2967/jnumed.110.085258.
- Chekmenev, Eduard Y. MRI "Hyperpolarization and Molecular Imaging" mi Gateway, Newsletter of the SNMMI CMIIT, Vol. 7, Issue 3, 2013-3
The suggested reading list was actually used to prepare this post. This was an introductory post in the realm of HP MRI imaging - hope you enjoyed it.
Cerebrovascular disease encompasses a range of pathologies that affect different components of the cerebral vasculature and brain parenchyma. Large artery atherosclerosis, acute cerebral ischaemia, and intracerebral small vessel disease all demonstrate altered metabolic processes that are key to their pathogenesis. Positron Emission Tomography (PET) can detect and quantify metabolic processes that are relevant to each facet of cerebrovascular disease. The review article published in the November 2017 issue of Nature Reviews Neurology describes how PET-based imaging of metabolic processes at the neurovascular interface has contributed to our understanding of cerebrovascular disease.
Evans, N. R. et al. PET imaging of the neurovascular interface in cerebrovascular disease. Nat Rev Neurol 13, 676–688 (2017). doi:10.1038/nrneurol.2017.129
PET imaging employs various radioligands to detect physiological processes in vivo. The article written by Nicholas R. Evans, University of Cambridge, Cambridge, UK and his colleagues summaries the radioisotopes of PET ligands used for the following list of cellular or physiological targets of vascular biology, actute ischaemic stroke and small vessel disease:
- Increased metabolic rate (inflammation): 18F-FDG
2. Macrophages: 68Ga-DOTATATE (targeting somatostatin receptor type 2)
3. Microcalcification: 18F-NaF (hydroapatite)
4. Hypoxia: 18F-FMISO (targeting selective reduction in hypoxia)
5. Macrophages and microglia: 11C-PK11195, 11C-PBR28, 18F-DPA-714, 11C-vinpocetine, 18F-GE-180 (all targeting TSPO)
6. Neurons: 11C-FMZ (GABA-A receptor)
7. Amyloid: 11C-PiB (analogue of thioflavin T)
8. Neurons: 18F-FNDP (epoxide hydrolase enzyme)
9. Expressed on neurons, astrocytes, microglia and endothelial cells: 18F-NS14490 (α7 nicotonic acetylcholine receptor)
10. Apoptosis: 18F-labeled isatins (caspase 3, caspase 7)
The review article considers sensitivity, specificity, technical considerations and also clinical implications for each radiotracersThe nanoScan PET/MRI3T is an ideal combination of modalities for research of cerebrovascular diseases: structural imaging provided by MRI is co-registered and combined with the PET ability to detect and quantify these pathophysiological processes in vivo. Information obtained from PET studies has helped to shape the understanding of key concepts in cerebrovascular medicine, including vulnerable atherosclerotic plaque, salvageable ischaemic penumbra, neuroinflammation and selective neuronal loss after ischaemic insult. New PET ligands continue to be developed that have superior specificity or that target new processes of interest.
18F-FDG-PET Detects Drastic Changes in Brain Metabolism in the Tg4–42 Model of Alzheimer’s Disease
Caroline Bouter1, Philipp Henniges2, Timon N. Franke2, Caroline Irwin2, Carsten Oliver Sahlmann1, Marius E. Sichler2, Nicola Beindorff3, Thomas A. Bayer2 and Yvonne Bouter2
1Department of Nuclear Medicine, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
2Division of Molecular Psychiatry, Department of Psychiatry and Psychotherapy, University Medical Center Göttingen (UMG), Georg-August-University, Göttingen, Germany
3Berlin Experimental Radionuclide Imaging Center (BERIC), Charité—University Medicine Berlin, Berlin, Germany
https://doi.org/10.3389/fnagi.2018.00425
Summary
The authors have used one of the latest mouse Alzheimer’s Disease (AD) models (Tg4-42 transgenic mutation, which overexpresses the Ab4-42 peptide, which is truncated on the N-terminal region, causing neurotoxicity and Ab aggregation, which are similar to AD). As the AD patients show altered glucose metabolism, the authors chose to follow it with the most common radiopharmaceutical used in PET, namely 18F-FDG, and how it can be used together with the MRI as an early biomarker for AD.
They have found that Tg4-42 mice show a reduction of glucose-metabolism, which correlates with their age, and the decreased 18F-FDG uptake can be shown in early age (3 months).
Results from nanoScan PET/MRI 1T
For the PET/MRI studies, the authors have used a nanoScan PET/MRI 1T, which could provide a fast measurement method together with good statistics. Young (3-4 months) and aged (7-8 months) Tg4-42 (n=7, female) and aged WT C57Bl/6J (7-8 months, n=5, female) control mice were used in this study. The authors have followed the standard 18F-FDG PET/MRI protocol, as the mice were fasted overnight, and 9-21 MBq activity was injected into the tail vein, with a 45 minute long uptake period.
The PET scans were performed for 20 minutes , which was followed by a Tera-Tomo 3D reconstruction method with a 0.3 mm3. For the MRI, the authors have used GRE sequence as a material map for attenuation and scatter correction in the PET reconstruction, and as a brain atlas. The analysis was performed with PMOD software.
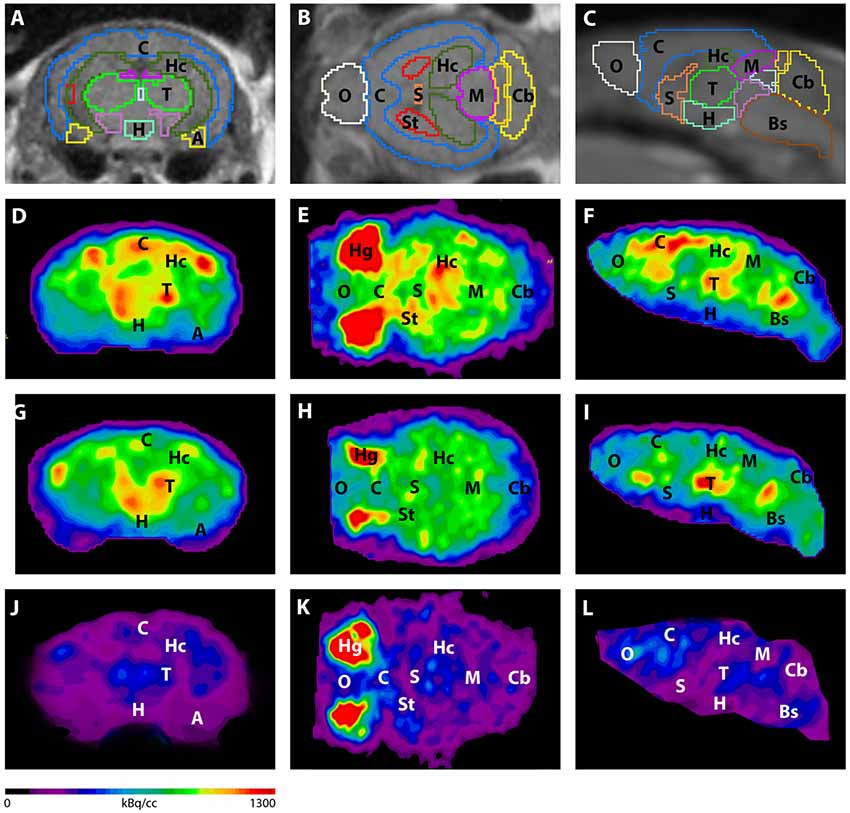
Figure 3. shows the main results from the PET/MRI acquisitions: a-c) MRI images were matched with predefined brain regions; axial, coronal and sagittal view. d-f) 18F-FDG-PET images of a WT mouse. g-i) 18F-FDG-PET images of a young Tg4–42 mouse. j-l) 18F-FDG-PET images of an aged Tg4–42 mouse. A, Amygdala; Bs, Brain Stem; C, Cortex; Cb, Cerebellum; H, Hypothalamus; Hc, Hippocampus; Hg, Harderian glands; M, Midbrain; O, Olfactory Bulb; S, Septum/Basal Forebrain; St, Striatum; T, Thalamus.
- These results suggest that 18F-FDG uptake was distinctly lower in aged Tg4–42 mice compared to WT mice. In young Tg4–42 mice 18F-FDG the uptake did not show significant differences in whole brain uptake but it was reduced in the hippocampus, forebrain, hypothalamus, amygdala and midbrain.
- The study showed that Tg4-42 can be a useful AD model to monitor the effects of various therapeutic strategies in vivo using 18F-FDG uptake as a therapeutic readout.